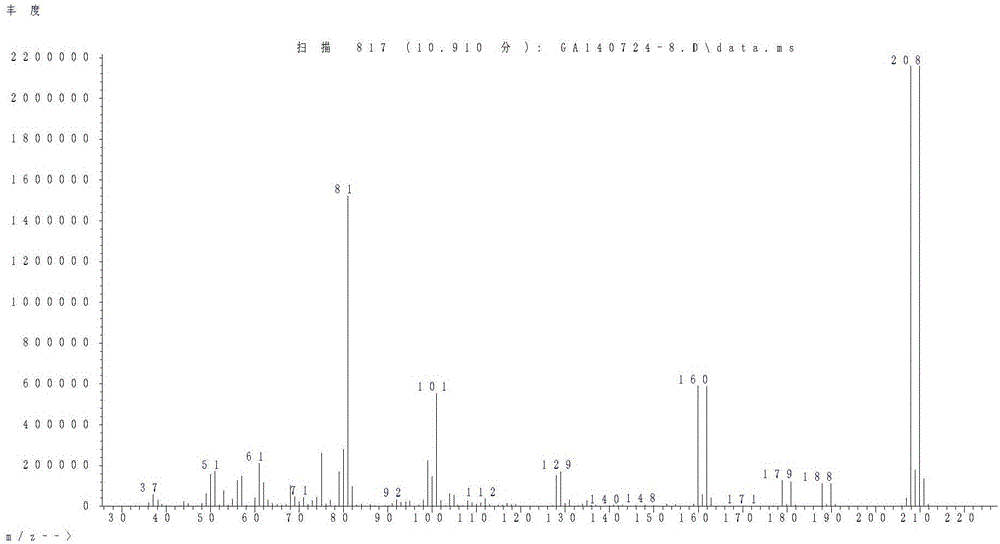
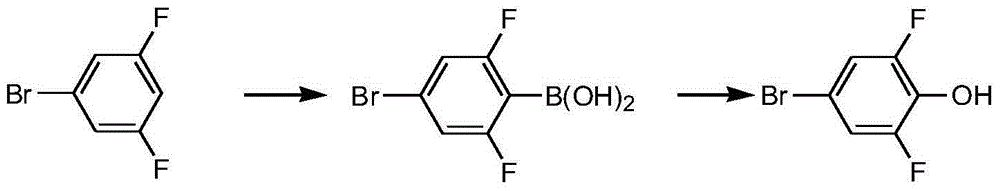
Preparing method of 2,6-difluoro-4-bromophenol
A technology of difluorobromobenzene and bromophenol, which is applied in the field of preparation of 2,6-difluoro-4-bromophenol, can solve the problems of product distillation, high production cost, and high safety risk, and achieve low cost and easy Effects of purchasing and reducing operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Under the protection of nitrogen, add 60.6g of diisopropylamine and 127g of methyl tert-butyl ether into a clean and dry three-necked flask, stir mechanically and lower the temperature to -55°C, and add 240ml of normal The n-hexane solution of butyllithium (the molar concentration of n-butyllithium is 2.5 mol / L), was kept at -55~-50°C for 0.5 hours after dropping, and the LDA reagent was obtained after the keeping.
[0025] 2) Under nitrogen protection, add 115.8g (0.6mol) of 3,5-difluorobromobenzene and 150g of methyl tert-butyl ether into a clean and dry three-necked flask, start stirring, and cool down to the inner temperature of -55°C. Add the LDA reagent obtained in 1) dropwise at an internal temperature of -55~-50°C, and keep warm at -55~-50°C for 2 hours after the dropwise addition;
[0026] 3) After the heat preservation is over, control the internal temperature of the system obtained in 2) to be -55~-50°C, add 81.2g (0.78mol) of trimethyl borate dropwise to ...
Embodiment 2
[0031] 1) Under the protection of nitrogen, add 60.6g of diisopropylamine and 120g of methyl tert-butyl ether into a clean and dry three-necked flask, stir mechanically and lower the temperature to -55°C, and add 240ml of secondary The n-hexane solution of butyllithium (the molar concentration of sec-butyllithium is 2.5 mol / L), was kept at -55~-50°C for 0.5 hours after dropping, and the LDA reagent was obtained after the keeping.
[0032] 2) Under nitrogen protection, add 115.8g (0.6mol) of 3,5-difluorobromobenzene and 150g of methyl tert-butyl ether into a clean and dry three-necked flask, start stirring, and cool down to the inner temperature of -55°C. Control the internal temperature at -55~-50°C and add the reaction solution in 1) dropwise, and keep it at -55~-50°C for 2 hours after dropping,
[0033] 3) After the heat preservation is completed, control the internal temperature of the system obtained in 2) to be -55~-50°C, add 146.6g (0.78mol) triisopropyl borate dropwise,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com