Porcine circovirus type 2 strain, inactivated vaccine prepared from porcine circovirus type 2 strain and application of porcine circovirus type 2 strain
A technology of porcine circovirus and virus liquid, which is applied in antiviral agents, virus/bacteriophage, virus antigen components, etc., can solve the problem of lack of PCV2 inactivated vaccine, etc., and achieve the effect of strong immune protection and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Isolation and Identification of Porcine Circovirus Type 2 Yh-1 Strain
[0035] 1. Experimental method
[0036] 1.1 Disease samples and cells for virus isolation
[0037]The lungs and lymph nodes of the dead piglets in a pig farm in Inner Mongolia were collected and stored in a -70°C ultra-low temperature freezer for later use. PK-15 cells are preserved by our research and development center, using GibcoMEM medium containing 10% calf serum and 5% CO at 37°C 2 Incubator cultivation.
[0038] 1.2 Treatment of disease materials
[0039] Cut a certain volume of disease material, add 5 times the volume of MEM with 5% serum, grind it with a glass grinder, centrifuge the homogenate at 12000rpm at 4°C for 10min, and filter the supernatant with a 0.22μm filter for sterilization before use.
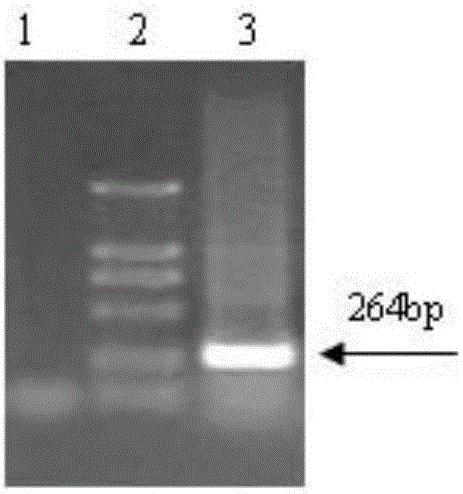
[0040] 1.3 Primer design
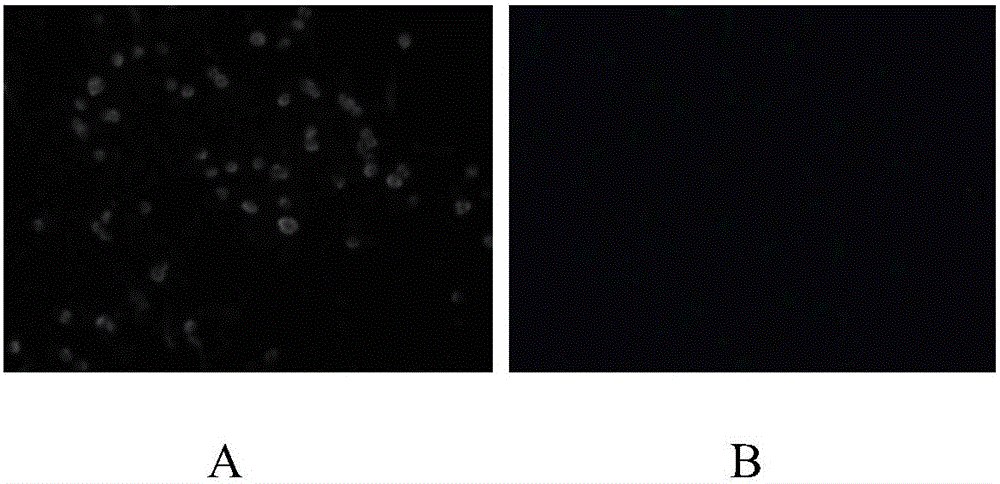
[0041] PCV2-specific detection primers were designed according to the published PCV2 sequence, the virus gene sequence was amplified by PCR method, s...
Embodiment 2
[0060] The preparation of the inactivated vaccine of embodiment 2 porcine circovirus type 2 Yh-1 strains
[0061] 1. Experimental method
[0062] 1.1 Virus inactivation
[0063] Add 0.2% of the total amount of PCV-2Yh-1 strain F15 virus solution into formaldehyde solution, shake for 5 minutes, then inactivate at 37°C for 20 hours, shake 3 to 4 times, 3 minutes each time. Store the inactivation solution at 2-8°C.
[0064] 1.2 Inactivation test
[0065] Take the inactivated virus solution of PCV-2Yh-1 strain, inoculate pk-15 cells that have grown into a monolayer by 50 times dilution, inoculate 4 bottles of each sample, 1 mL per bottle, after adsorption for 1 hour, add maintenance solution to the original volume at 37°C Continue to culture for 4 days, there should be no cytopathic changes; continue to culture, and then blind passage for 2 generations, and then use indirect immunofluorescence and PCR to detect the virus. At the same time, the same batch of uninactivated virus...
experiment example 2
[0086] Experimental Example 2 Safety Test of Porcine Circovirus Type 2 Inactivated Vaccine
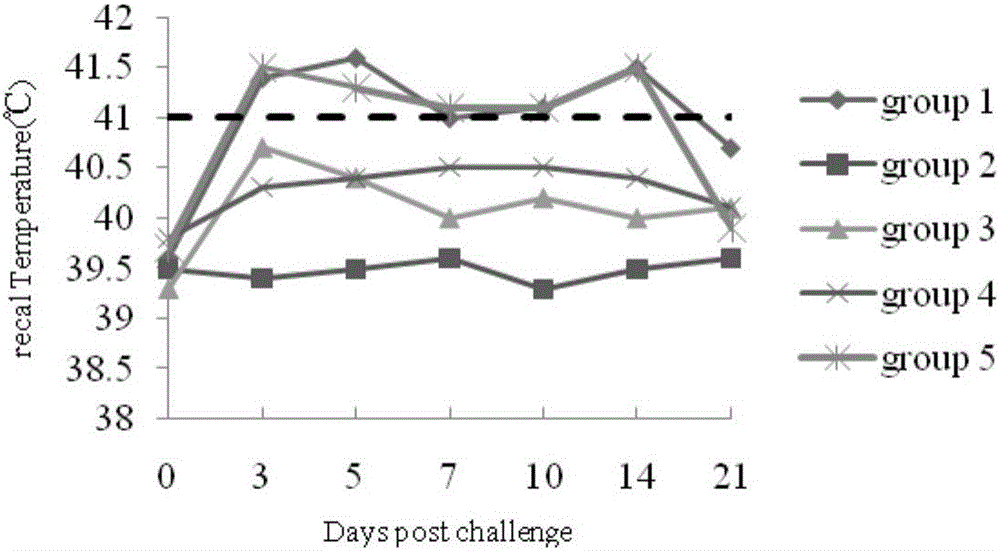
[0087] Fifteen PRRSV-negative piglets aged 4-5 weeks were randomly divided into 3 groups, 5 piglets / group, group I received a single dose of inactivated vaccine, group II received a double dose of inactivated vaccine, and group III was injected with emulsified MEM medium 2 mL was used as a control. Measure the body temperature before immunization, and measure the body temperature every day for 7 days after immunization; observe the feeding situation of the pigs for 14 days after immunization, touch the local area, and see if there is any lump. 28 days after immunization, 2 pigs in each group were dissected to observe the absorption of the vaccine.
[0088]The body temperature of pigs before and after immunization with single dose and double dose was measured. There was no phenomenon of elevated body temperature in pigs before and after immunization. The pigs are fed and mentally nor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com