Method for producing isocyanate
一种异氰酸酯、制造方法的技术,应用在异氰酸酯的制造领域,能够解决处理繁杂、毒性强等问题,达到回收容易的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
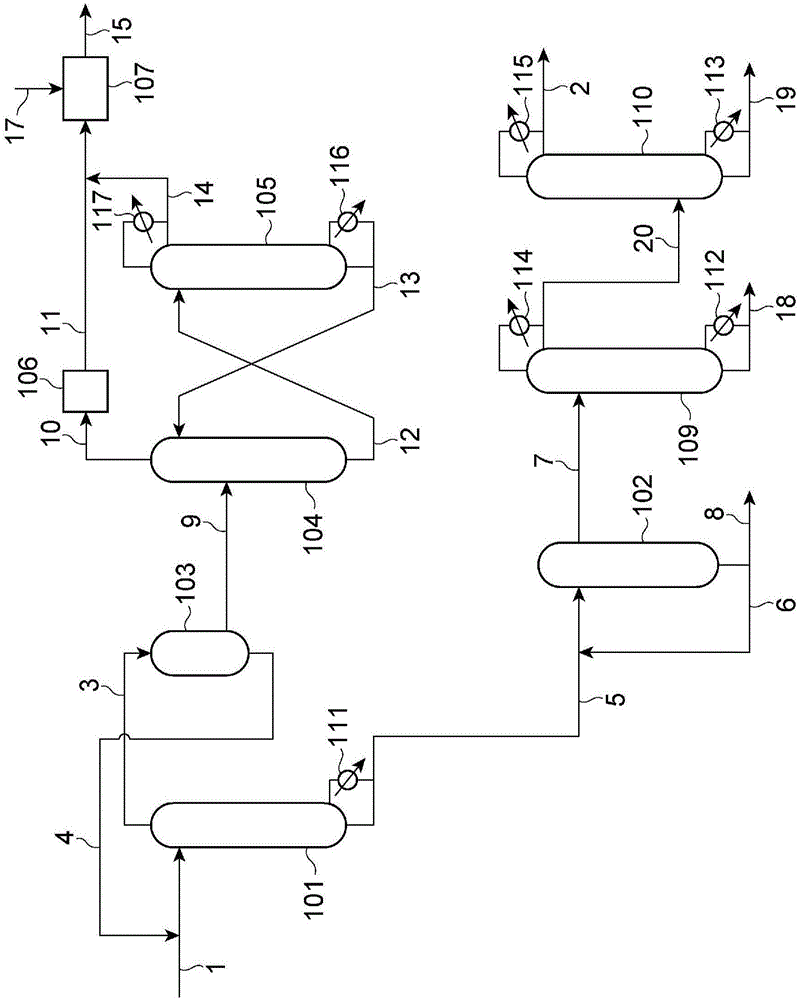
[0752] use figure 1 device shown. figure 1 The apparatus shown is an apparatus for performing a urethanization step, a step of separating a gas phase component mainly composed of ammonia, a step of manufacturing a gas-absorbing liquid, an ammonia separation step, a urea manufacturing step, and an isocyanate manufacturing step.
[0753] 7.2 kg of 1,6-hexamethylenediamine, 7.5 kg of urea (out of 7.5 kg of urea, 5.3 kg is urea produced by the urea production facility 107 described later), 4-(1,1,3, A mixture of 261.9 kg of 3-tetramethylbutyl)phenol was supplied to the continuous multi-stage distillation column 101 at about 92.2 kg / Hr. The liquid mixture of 4-(1,1,3,3-tetramethylbutyl)phenol and urea obtained in the condenser 103 was supplied from the line 4 at about 9.56 kg / Hr. The continuous multi-stage distillation column 101 is an apparatus for performing the urethanization step, and the temperature at the bottom of the column is 250° C. and the pressure at the top of the co...
Embodiment 2
[0758] (urethane process)
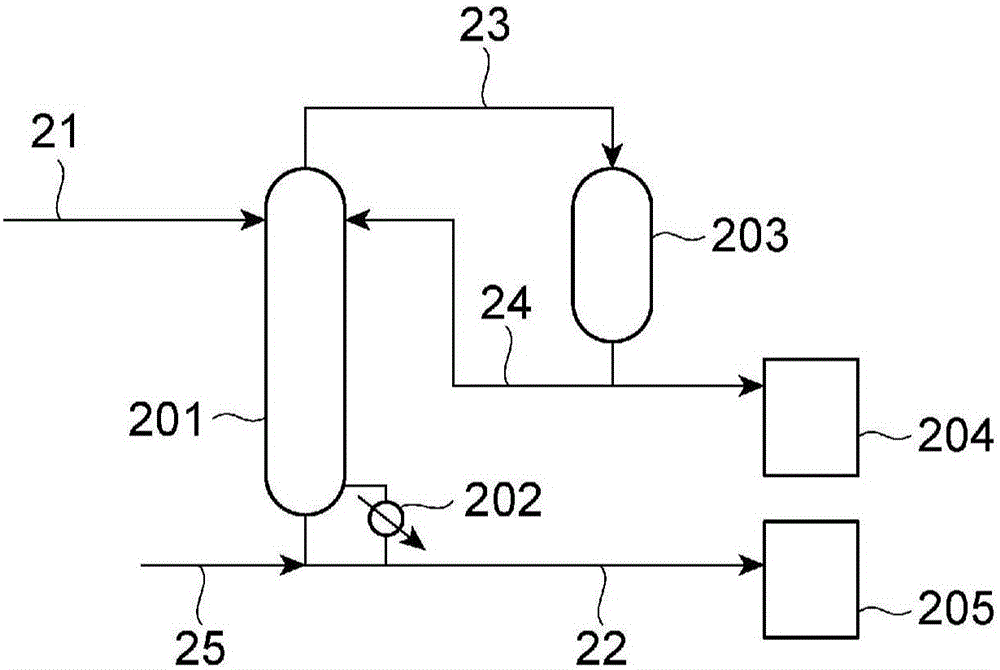
[0759] use figure 2The apparatus shown performs the urethanization process. A mixture of 11.3 kg of 1-amino-3-aminomethyl-3,5,5-trimethylcyclohexane, 15.1 kg of urea, and 220.3 kg of 1-butanol is supplied to the continuous multi-stage via line 21 at about 20 kg / Hr Distillation column 201. The mixed solution of 1-butanol and urea obtained in the condenser 203 was supplied from the line 24 at about 3.5 kg / Hr. The remaining condensed components are recovered to the storage tank 204 . The continuous multi-stage distillation column 201 is a device for performing the urethanization step, and the temperature at the bottom of the column is 220° C. and the pressure at the top of the column is 1.2 MPa by heating with the reboiler 202 . The reaction liquid is withdrawn from the bottom of the continuous multi-stage distillation column 201 and recovered to the storage tank 205 through the pipeline 22 .
[0760] (Preliminary Concentration Process)
[0761]...
Embodiment 3
[0772] (urethane process)
[0773] Use 4,4'-dicyclohexylmethanediamine 21kg instead of 1-amino-3-aminomethyl-3,5,5-trimethylcyclohexane, use urea 16.5kg, use 2,4-xylenol 250 kg was replaced with 1-butanol, and the method similar to the urethanization process of Example 2 was performed except having set the tower bottom temperature to 240 degreeC. From figure 2 The reaction liquid is extracted from the bottom of the continuous multi-stage distillation column 201, and is recovered to the storage tank 205 through the pipeline 22.
[0774] (Preliminary Concentration Process)
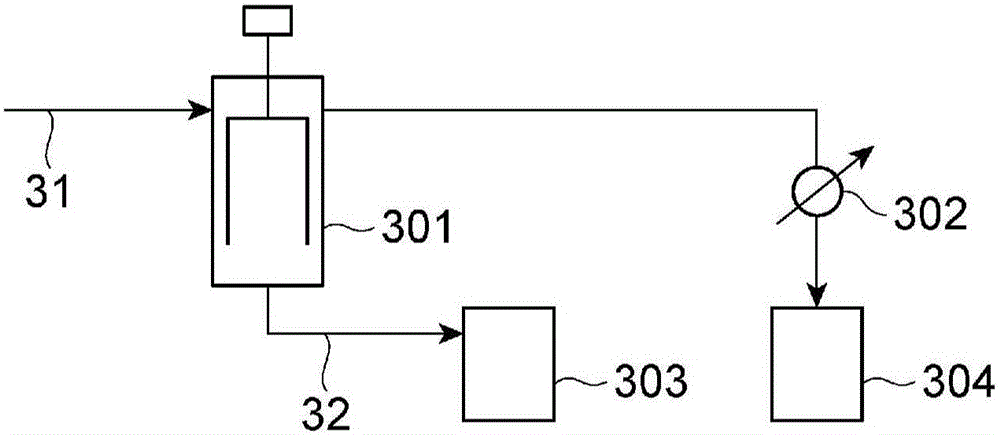
[0775] Make image 3 The jacket temperature of the thin-film evaporator 301 is 150°C, and the internal pressure is 10kPa. In addition, the same method as the preliminary concentration process of Example 2 is carried out, and the liquid is recovered from the bottom of the thin-film evaporator 301 at about 10kg / Hr. phase composition.
[0776] (Thermal decomposition process)
[0777] use Figure 4 The s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com