Traditional Chinese medicine composition for treating viral myocarditis and preparation method thereof
A technology for viral myocarditis and composition, applied in the field of traditional Chinese medicine composition for the treatment of viral myocarditis and its preparation field, can solve the problems of unsatisfactory clinical effect, large toxic and side effects of western medicine, limited effect of medication guidance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a preparation method of the above-mentioned traditional Chinese medicine composition, comprising:
[0043] Mix the components in the raw material components in proportion, pulverize them, soak them in 5-8 times the amount of water for 12-24 hours, extract the volatile oil by steam distillation, save the distilled medicinal residues and medicinal liquid separately, and reduce the medicinal liquid Press recovery and concentrate until the relative density is 1.07-1.13 at 75-85°C to obtain A; add 65% ethanol to dissolve the above-mentioned volatile oil at a ratio of 1:1, and use colloidal milling method to make the ratio of volatile oil and β-cyclodextrin 1:7 -9, The ratio of β-cyclodextrin to water is 1:1-3, adopt appropriate grinding speed to grind for 15-30 minutes for clathrate, obtain paste, dry at 40-60°C, and pulverize to obtain clathrate B; the above dregs continue to be reflux extracted with 65-85% ethanol for 1-3 times, 5-10 tim...
Embodiment 1
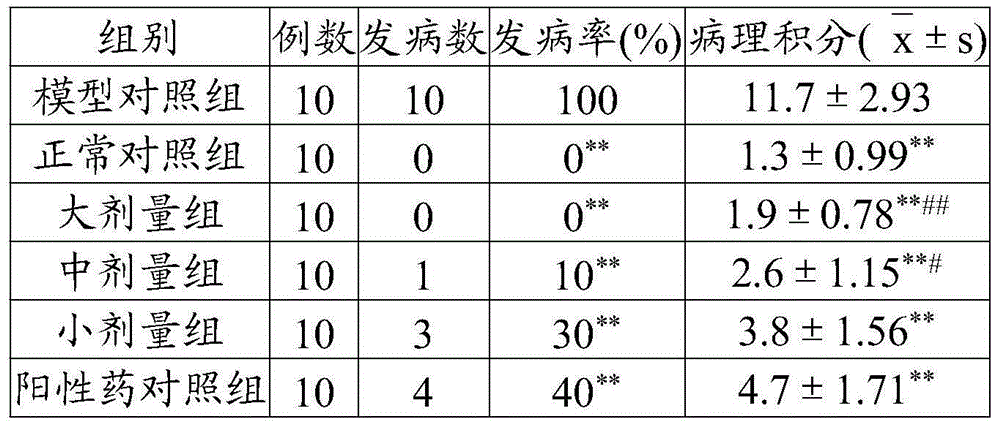
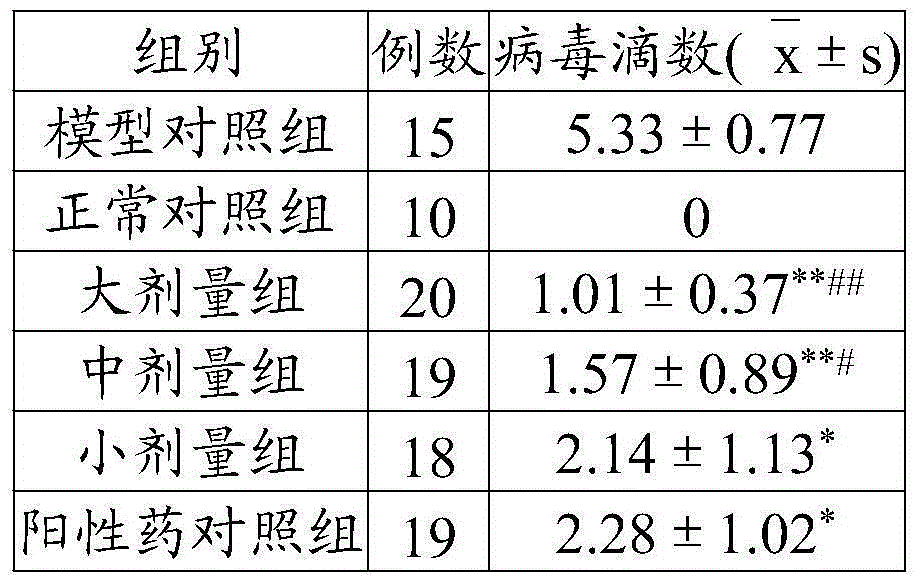
[0048] Take 10 parts by weight of Sophora japonica, 8 parts by weight of Schisandra chinensis, 6 parts by weight of notopterygium, 8 parts by weight of rehmannia glutinosa, 5 parts by weight of epimedium, 10 parts by weight of honeysuckle, 9 parts by weight of cassia twig, 6 parts by weight of hawthorn, and 5 parts by weight of longan meat 6 parts by weight of Cassia Spatholobus, 8 parts by weight of Cassia Semen, 8 parts by weight of Ophiopogon japonicus, 10 parts by weight of Ligustrum lucidum, 6 parts by weight of Angelica sinensis, 5 parts by weight of Rhizoma Chuanxiong, mixed in proportion, pulverized; hours, extract the volatile oil by steam distillation, save the distilled medicinal residues and medicinal liquid, recover and concentrate the medicinal liquid under reduced pressure until the relative density is 1.07 at 75°C to obtain A; the above-mentioned volatile oil is dissolved in 65% ethanol at a ratio of 1:1 Adopt the colloid milling method, take the ratio of volati...
Embodiment 2
[0052] Take 16 parts by weight of Sophora japonica, 14 parts by weight of Schisandra chinensis, 12 parts by weight of notopterygium, 15 parts by weight of rehmannia glutinosa, 10 parts by weight of epimedium, 16 parts by weight of honeysuckle, 18 parts by weight of cassia twig, 14 parts by weight of hawthorn, and 10 parts by weight of longan meat 12 parts by weight of Cassia Spatholobus, 18 parts by weight of Cassia Semen, 14 parts by weight of Ophiopogon japonicus, 16 parts by weight of Ligustrum lucidum, 12 parts by weight of Angelica sinensis, 10 parts by weight of Rhizoma Chuanxiong, mixed in proportion, pulverized; soaked in 8 times the amount of water for 24 hours, extract the volatile oil by steam distillation, save the distilled medicinal residues and medicinal liquid, recover and concentrate the medicinal liquid under reduced pressure until the relative density is 1.13 at 85°C to obtain A; the above-mentioned volatile oil is dissolved by adding 65% ethanol in a ratio of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com