Retinal pigment epithelium epithelial-mesenchymal transition model and application thereof
A retinal pigment and epithelial cell technology, applied in the field of retinal pigment epithelial cell epithelial-mesenchymal transition model, can solve the problems that the cell model cannot be widely used, and the connection between cells is difficult to be destroyed. The method is simple and easy to implement , good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
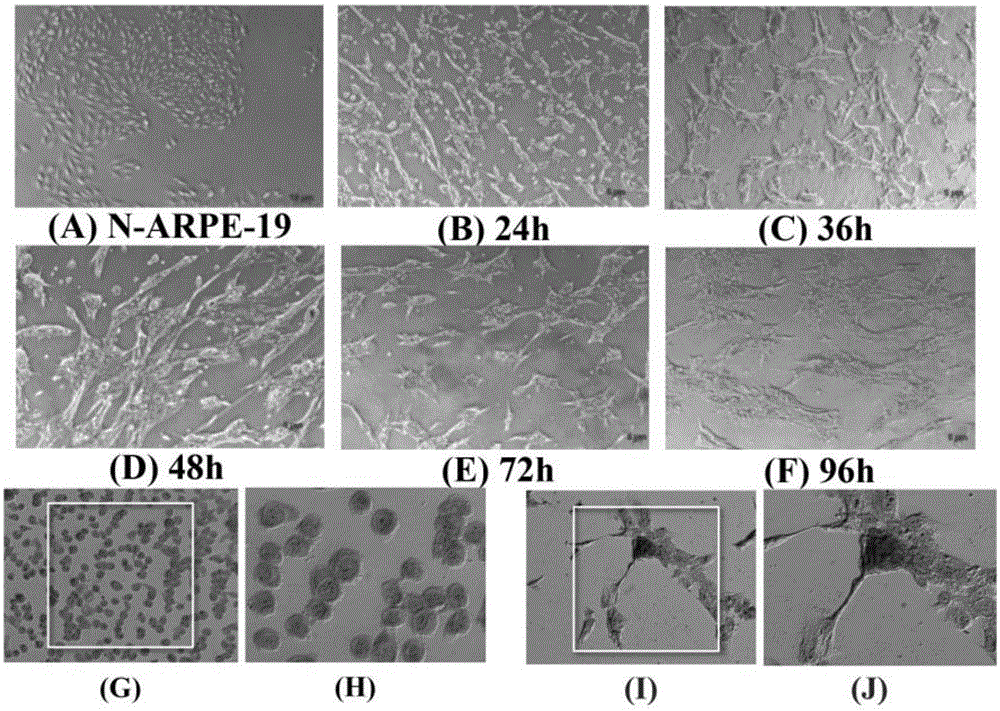
[0037] Cell culture:
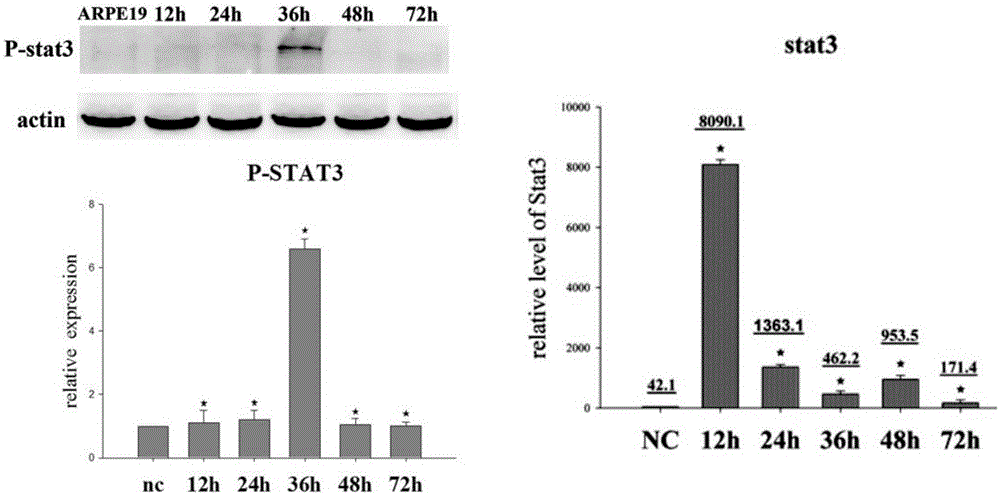
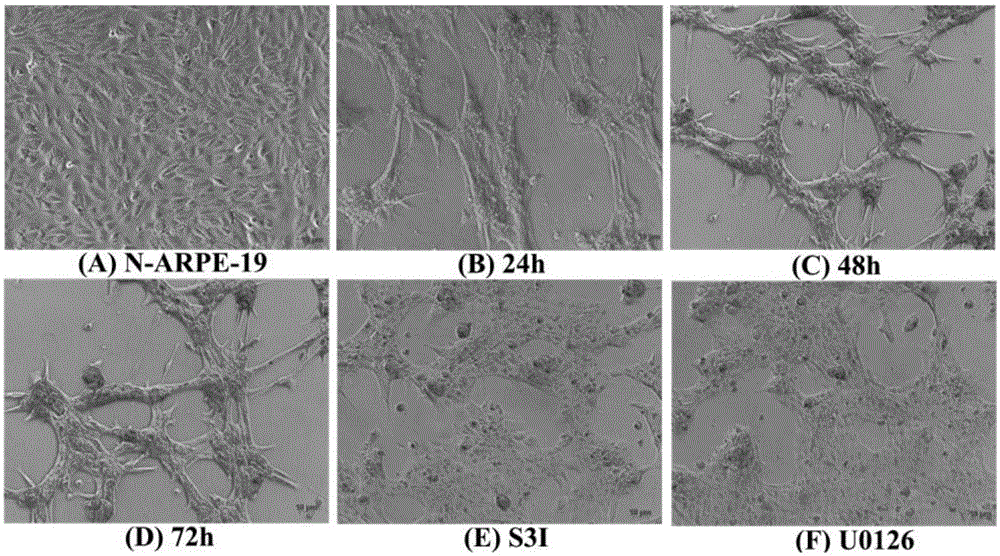
[0038] The culture of ARPE-19 cells was divided into two parts: in the cell dish and in the EMT model. When cultured in a cell dish, culture in DMEM-F12 medium containing 10% fetal bovine serum (FBS) and 1% penicillin-streptavidin double antibody. Cells per dish (10cm culture dish, cell density is 1×107cells / dish). When doing EMT model, ARPE-19 cells were digested with trypsin, and then cultured in serum-free DMEM-F12 medium containing N2 and non-essential amino acids (NEAA) in a bacterial dish, at 12h and 24h respectively After 36h, 48h, and 72h, cell samples were collected or dissolved in trizol, and detected by Q-PCR, ELISA, Western blot and other methods. The cells in two dishes were added with S3I (Stat3 inhibitor) and U0126 respectively at 24h, and the samples were collected at 48h. In the experiment of detecting stat3 activation, the P-STAT3-TA-LUC reporter gene plasmid was first transfected into ARPE-19 with lipofectaminer2000, and after 48 ho...
Embodiment 2
[0053] To investigate the inhibitory effect of mir-24 on the EMT of ARPE-19.
[0054] 1. Cell culture
[0055] The main body of cell culture is mir-24-ARPE-19 cells, which are divided into two parts, one of which is to verify the effect of mir-24 on the EMT, proliferation and migration of ARPE-19 cells, as well as the effect of stat3 signaling pathway. One part is to clarify the targeting effect of mir-24 and inflammatory factor CHI3L1.
[0056] The details are as follows: Validation of the effect of mir-24 on the EMT of ARPE-19 cells: cultured in serum-free DMEM / F12 containing N2 and NEAA, petridish. To verify the effect of mir-24 on the proliferation and migration of ARPE-19 cells: culture in DMED / F12 culture medium with 10% fetal bovine serum, celldish, scratch after 24 hours, change to serum-free culture medium and continue to culture. To verify the effect of mir24 on the stat3 signaling pathway in the EMT process of ARPE-19 cells: cultured in DMED / F12 medium with 10% fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com