Method for targeted modification of algae genomes
A targeted modification and genome technology, applied in the field of genetic material in modified algae cells, can solve problems such as low conversion rate and weak transgene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Increased frequency of targeted mutagenesis at endogenous loci using the PTRI20 meganuclease
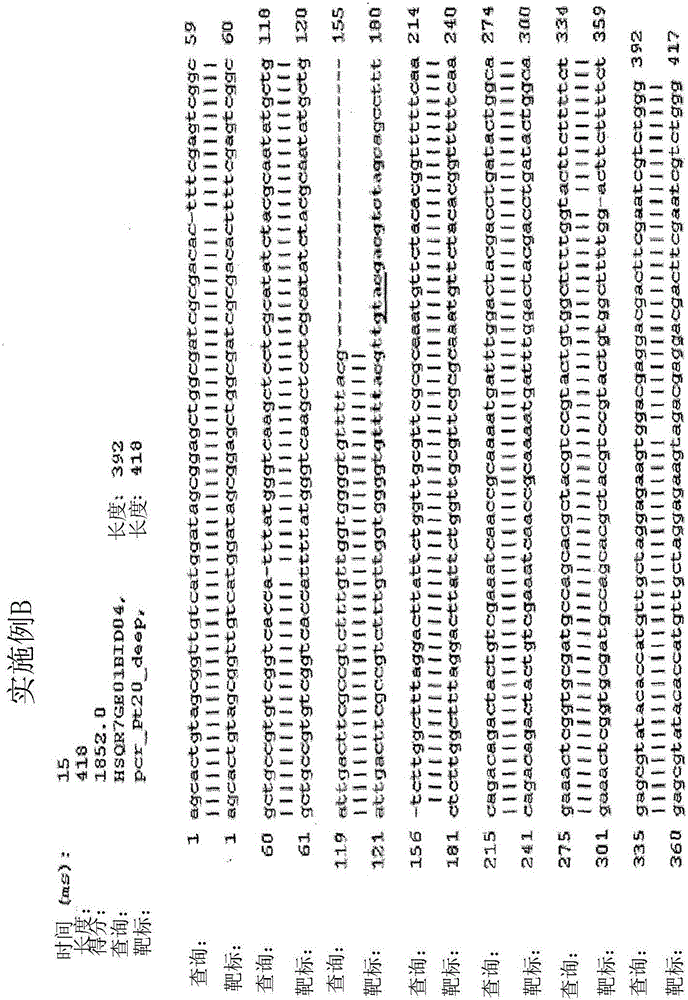
[0108] To investigate the ability of a meganuclease to increase the frequency of targeted mutagenesis at an endogenous locus in diatoms, an engineered meganuclease ( known as PTRI20), which was designed to cleave the DNA sequence 5'-GTTTTACGTTGTACGACGTCTAGC-3' (SEQ ID NO: 2). A plasmid encoding a meganuclease and a plasmid encoding a selection gene (Nat1 ) (SEQ ID NO: 3) were co-transformed into diatoms. Mutagenesis rates were measured by deep sequencing on individual clones resulting from transformation.
[0109] Materials and methods
[0110] Culture conditions
[0111] In a Sanyo incubator (model MLR-351) at a constant temperature (20 + / - 0.5°C) on silica-free filtered Guillard's f / 2 medium (40° / °° w / v SigmaSeaSaltsS9883) Phaeodactylum tricornutum Bohlin clone CCMP2561 was grown in medium supplemented with 1X Guillard's f / 2 concentrated seawater solution ...
Embodiment 2
[0125] Example 2: Using a combination of SCTREX2 and PTRI20 meganucleases at diatom endogenous loci High frequency of targeted mutagenesis
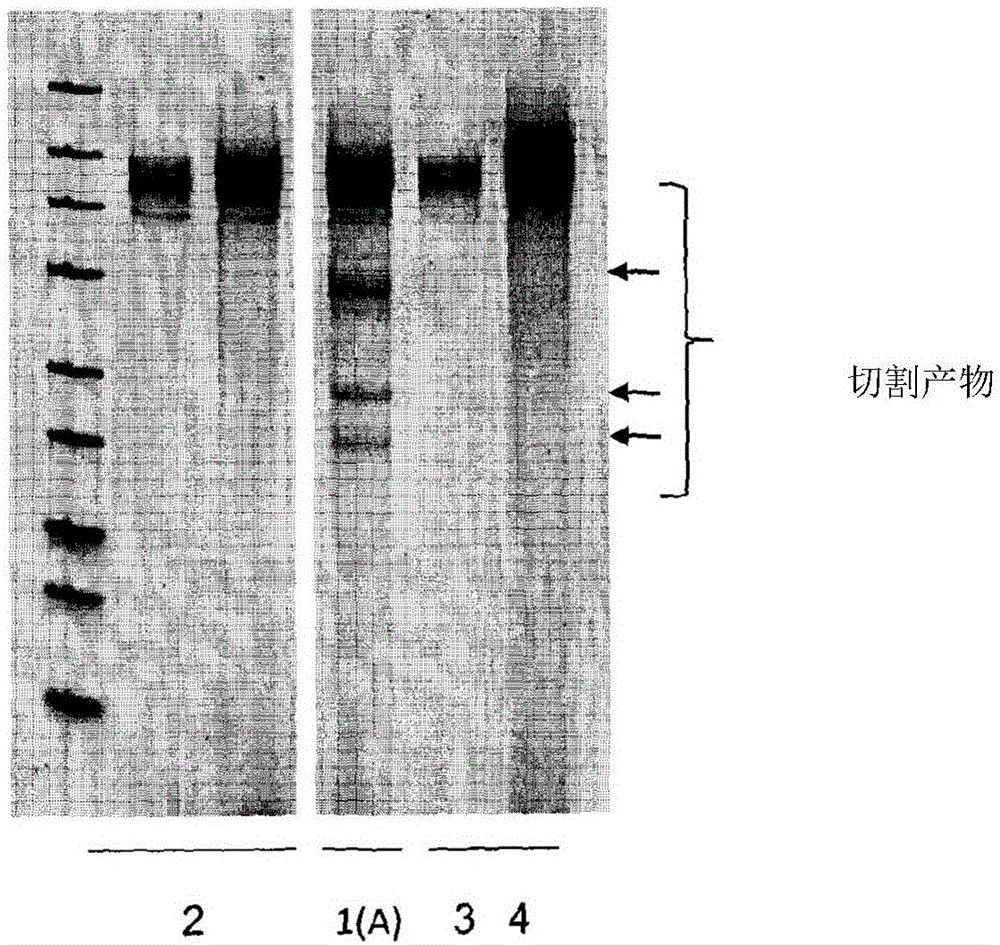
[0126] To investigate the ability of the DNA processing enzyme single-stranded TREX2 (SCTREX2) to increase the frequency of targeted mutagenesis induced by meganucleases, an engineered meganuclease encoded by the pCLS17038 plasmid (SEQ ID NO: 1) was used (referred to as PTRI20), which is designed to cleave DNA 5'-GTTTTACGTTGTACGACGTCTAGC-3' (SEQ ID NO: 2). This meganuclease was co-transformed with a plasmid encoding a selection gene (Nat1) (NAT) (SEQ ID NO: 3), and a plasmid encoding a DNA processing enzyme called SCTREX2, encoded by pCLS18296 (SEQ ID NO: 7). Mutagenesis rates were visualized by T7 analysis, measured by deep sequencing on individual clones resulting from transformation.
[0127] Materials and methods
[0128] Phaeodactylum tricornutum Bohlin clone CCMP2561 was cultured according to the method described in Example 1 ...
Embodiment 3
[0144] Example 3: Using a combination of SCTREX2 and PTRI02 meganucleases at diatom endogenous loci High frequency of targeted mutagenesis
[0145]To investigate the ability of the DNA processing enzyme SCTREX2 to increase the frequency of targeted mutagenesis induced by meganucleases, an engineered meganuclease (termed PTRI02) encoded by the pCLS17181 plasmid (SEQ ID NO: 12), which Designed to cleave the DNA sequence 5'-TTTTGACGTCGTACGGTGTCTCCG-3' (SEQ ID NO: 13). A plasmid encoding this meganuclease was co-transformed with a plasmid encoding a selection gene (Nat1) (SEQ ID NO: 3), and a plasmid encoding a DNA processing enzyme, SCTREX2 (encoded by pCLS18296 (SEQ ID NO: 7)). Mutagenesis rates were measured by deep sequencing on individual clones resulting from transformation.
[0146] Materials and methods
[0147] Phaeodactylum tricornutum Bohlin clone CCMP2561 was cultured according to the method described in Example 1 and transformed with M17 tungsten particles (1.1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com