Method for synthesizing ketone compound
A technology of ketone compounds and synthesis methods, which is applied in the field of synthesis of ketone compounds, can solve the problems of application limitations, poor atom economy, cumbersome separation and purification process, etc., and achieve the goal of improving atom economy, avoiding resource waste, and avoiding environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011]
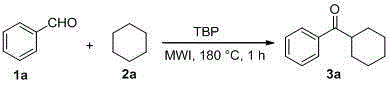
[0012] Add benzaldehyde ( 1a ,0.5mmol,53mg), cyclohexane ( 2a , 10mL) and di-tert-butyl peroxide (TBP, 2mmol, 380uL), then the pressure tube was sealed and placed in a microwave reactor, and the reaction was stirred at 180°C for 1 hour under microwave radiation. After the reaction is over, unreacted 2a , and the residue was separated through a silica gel column (petroleum ether / ethyl acetate=100 / 1) to obtain the product 3a (54.5mg, 58%). The characterization data of this compound are as follows: 1 HNMR (400MHz, CDCl 3 )δ:1.27-1.56(m,5H),1.74-1.77(m,1H),1.84-1.92(m,4H),3.28(tt, J 1 =11.2Hz, J 2 =3.2Hz,1H),7.47(t, J =7.6Hz,2H),7.54-7.58(m,1H),7.95-7.97(m,2H). 13 CNMR (100MHz, CDCl 3 )δ: 25.9, 26.0, 29.4, 45.6, 128.3, 128.6, 132.7, 136.4, 203.9. MS: m / z211[MNa] + .
Embodiment 2
[0014] Add benzaldehyde ( 1a ,0.5mmol,53mg), cyclohexane ( 2a , 10mL) and di-tert-butyl peroxide (TBP, 1mmol, 190uL), then the pressure-resistant tube was sealed and placed in a microwave reactor, and stirred and reacted at 180°C for 1 hour under microwave radiation. After the reaction is over, unreacted 2a , and the residue was separated through a silica gel column (petroleum ether / ethyl acetate=100 / 1) to obtain the product 3a (42.3 mg, 45%).
Embodiment 3
[0016] Add benzaldehyde ( 1a ,0.5mmol,53mg), cyclohexane ( 2a , 10mL) and di-tert-butyl peroxide (TBP, 3mmol, 570uL), then the pressure-resistant tube was sealed and placed in a microwave reactor, and reacted at 180°C for 1 hour under microwave radiation. After the reaction is over, unreacted 2a , and the residue was separated through a silica gel column (petroleum ether / ethyl acetate=100 / 1) to obtain the product 3a (51.7 mg, 55%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com