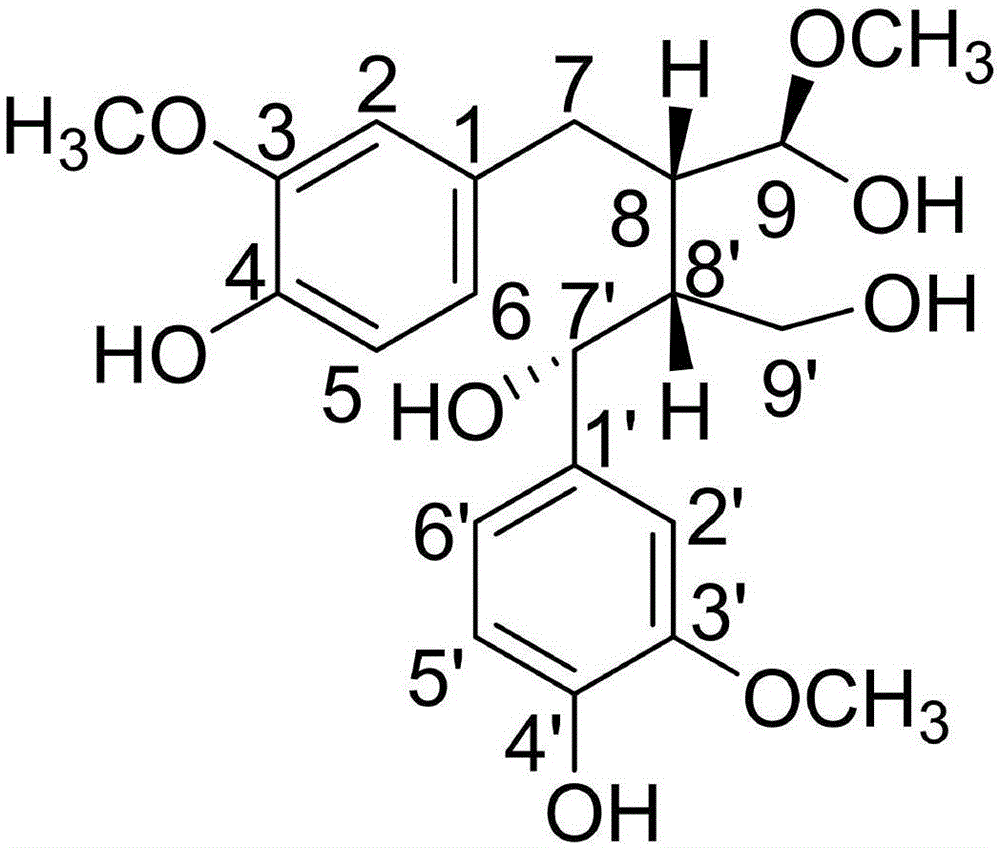
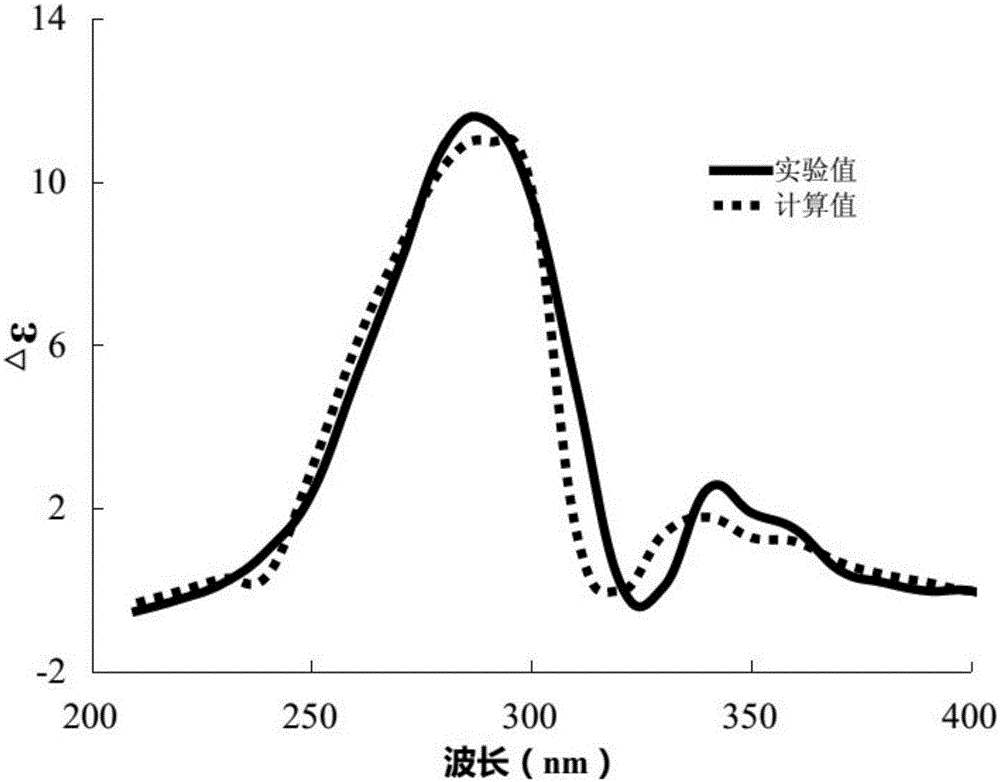
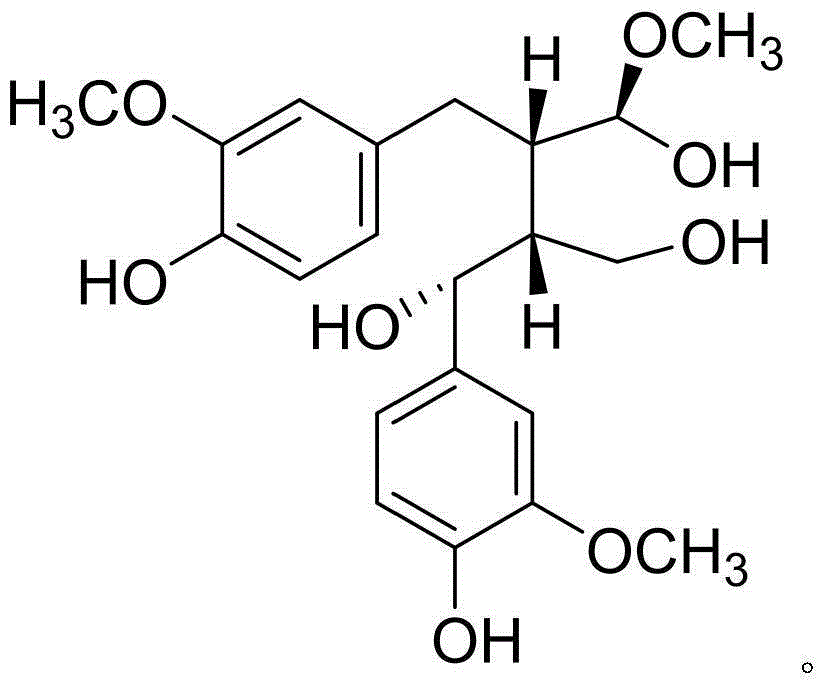
New lignan compounds, and preparation method and medical application thereof
A compound and drug technology, applied in the field of lignan compounds and their preparation, can solve the problems such as no medicinal records of Magnolia officinalis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Compound (I) Separation Preparation and Structure Confirmation
[0023] Sources of reagents: ethanol, petroleum ether, ethyl acetate, n-butanol, and dichloromethane were of analytical grade, purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Methanol, of analytical grade, were purchased from Jiangsu Hanbang Chemical Reagent Co., Ltd.
[0024] Preparation method: (a) crush the dry bark (8kg) of Magnolia officinalis, extract with 85% ethanol under heat reflux (25L×3 times), combine the extracts, concentrate to no alcohol smell (6L), and use petroleum ether ( 6L×3 times), ethyl acetate (6L×3 times) and water-saturated n-butanol (6L×3 times) were extracted to obtain petroleum ether extract, ethyl acetate extract (355g) and n-butanol extract (b) In the step (a), the ethyl acetate extract is removed with AB-8 type macroporous resin, first eluted with 15% ethanol for 6 column volumes, then with 75% ethanol for 10 column volumes, and collected 75% ethanol e...
Embodiment 2
[0026] Embodiment 2: compound (I) pharmacological action test
[0027] 1. Materials and Instruments
[0028] Vascular endothelial cells (ECV-304) were donated by the Department of Immunology, School of Medicine, Shandong University. Compound (I) is self-made, the preparation method is shown in Example 1, and the HPLC normalized purity is greater than 98%. RPMI-1640 dry powder medium and trypsin were purchased from Gibeo, USA. Fetal bovine serum was purchased from Tianjin TBD Company. MTT, agarose, and AnnexinV-FITC were purchased from Sigma, USA. LDH, MDA, SOD, GSH-Px assay kits were purchased from Nanjing Jiancheng Bioengineering Institute. DMSO was purchased from China Pharmaceutical (Group) Shanghai Chemical Reagent Co., Ltd. Hematoxylin was purchased from Fuzhou Maixin Biotechnology Co., Ltd. Rhodamine123 was purchased from Solarbic Corporation of the United States. The positive drug ligustrazine was purchased from Shanghai Yuanye Biotechnology Co., Ltd.
[0029] I...
Embodiment 3
[0064] Preparation of tablets: firstly prepare compound (I) according to the method of Example 1, and utilize organic acids such as tartaric acid, or citric acid, formic acid or oxalic acid, etc., inorganic acids such as hydrochloric acid or sulfuric acid or phosphoric acid to make salts, according to its The weight ratio of the excipient to the excipient is 1:9, and the excipient is granulated and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com