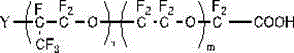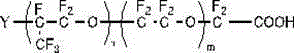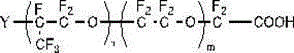Fluorosurfactant free of perfluorooctanoic acid as well as preparation method and process system of fluorosurfactant
A surfactant, perfluorooctanoic acid technology, applied in the field of fluoropolymers and their synthesis, can solve the problems that chemical characteristics cannot be characterized by any molecular chain or group in its structural formula, vary widely, and are magnified
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] This embodiment proposes a fluorine-containing surfactant that does not contain perfluorooctanoic acid. A repeating structural unit with a carboxyl group or a carboxylate salt at one end consisting of one or more combinations of the Y group selected from , R=halogen, the fluorosurfactant satisfies:
[0142] Acid value: 148mg / g;
[0143] Peroxygen value: 0.001%;
[0144] Boiling point: 130°C;
[0145] Surface tension: 10mN / m;
[0146] Critical micelle concentration: 0.05%;
[0147] Perfluorooctanoic acid and its salts: 0.
Embodiment 2
[0149] The fluorine-containing surfactant involved in this embodiment is a band at one end consisting of at least one oxyperfluoropropylene group and one or more combinations selected from perfluoromethoxy, Y groups, and oxyperfluoroethylene groups. The repeating structural unit of carboxyl or carboxylate, the Y group is selected from , R=hydrogen, the fluorosurfactant satisfies:
[0150] Acid value: 120mg / g;
[0151] Peroxide value: 1%;
[0152] Boiling point: 300°C;
[0153] Surface tension: 30mN / m;
[0154] Critical micelle concentration: 5%;
[0155] Perfluorooctanoic acid and its salts: 0.
Embodiment 3
[0157] The structure of the fluorosurfactant involved in the present embodiment is:
[0158]
[0159] Among them, the Y group is selected from , R=halogen,
[0160] Based on the molecular weight of the polymer, the content of halogen or hydrogen: 1%, n: 1, m / n: 0.01.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com