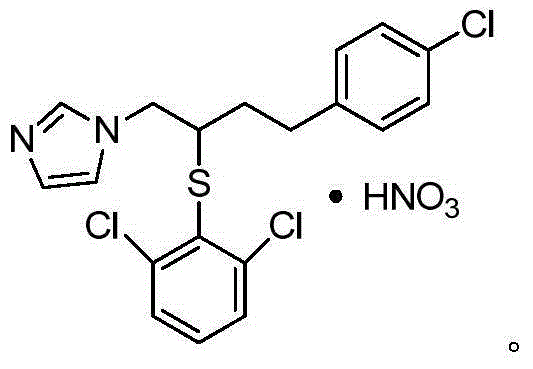
Method for industrially synthesizing butoconazole nitrate intermediate
A technology of butoconazole nitrate and intermediates, which is applied in the field of synthesis technology of butoconazole nitrate, can solve the problems that the product yield and purity cannot meet industrial production, the production process of butoconazole nitrate is complicated, and the cost is high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
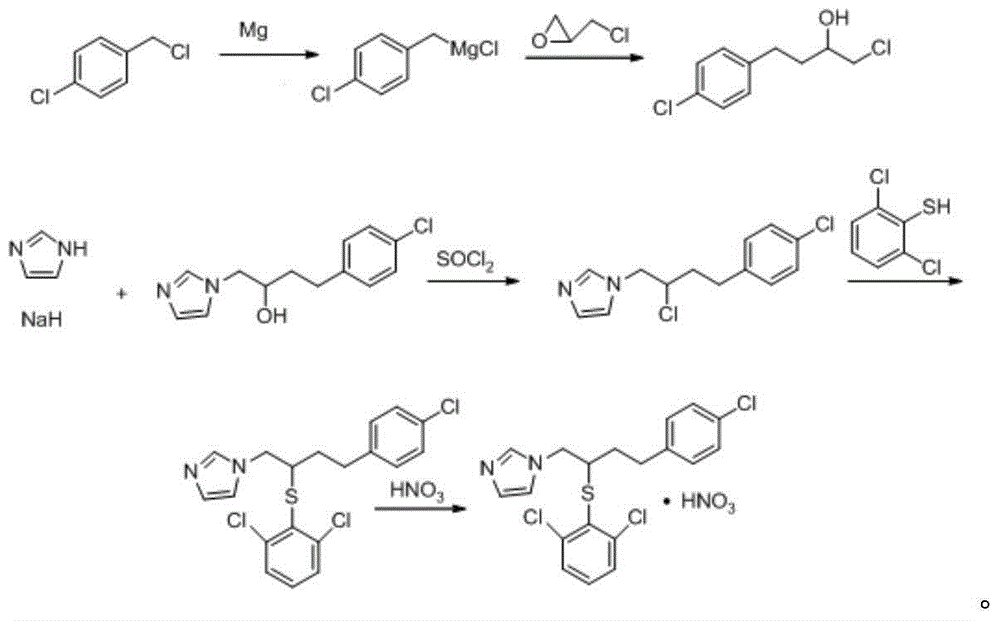
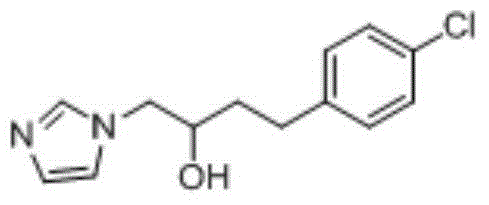
[0049] 1-(2-Hydroxy-4-(4-chlorophenyl)butyl)-1hydro-imidazole was prepared as follows:
[0050] (1) Get 7kg concentration of 20% sodium hydride DMF solution, under ice bath condition, add dropwise 7kg concentration of 20% imidazole DMF solution while stirring at a speed of 2ml / s, and stir and react at 60°C for 60min; After cooling in an ice-salt bath, slowly add 5 kg of 1-chloro-4-(4-chlorophenyl)-2-butanol, stir and react at 60°C for 120 minutes, and cool in an ice-salt bath to obtain a reaction solution;
[0051] (2) Get the reaction solution gained in step (1), add 25% n-hexane accounting for the weight of the reaction solution, stir at a speed of 3 rpm for 15 min, then add ice water accounting for 350% of the weight of the reaction solution, Stir at a speed of 3 revolutions per second until the precipitation stops and then filter, wash the filter cake once with water accounting for 1 / 3 times the weight of the filter cake, and dry it by centrifugation at a speed of 2825 r / m...
Embodiment 2
[0054] Compared with Example 1, the difference is only that the step (2) is specifically:
[0055] (2) Get the reaction solution gained in step (1), add 20% n-hexane accounting for the weight of the reaction solution, stir at a speed of 1 rpm for 10 min, then add ice water accounting for 300% of the weight of the reaction solution, Stir at a speed of 1 revolution / second until the precipitation stops and then filter, wash the filter cake once with water accounting for 1 / 3 times the weight of the filter cake, centrifuge and dry at a speed of 2825r / min for 50min, Ethyl acetate and activated carbon with 2 times and 0.04 times the weight of the product obtained, stood at -7°C for 10 hours for recrystallization, and dried the recrystallized product at 45°C to obtain the product.
[0056] After testing, the yield of the product of this example was 69.31%. The target product and impurity content were detected by HPLC and standard substances. After testing, the content of the target ...
Embodiment 3
[0058] Compared with Example 1, the difference is only that the step (2) is specifically:
[0059] (2) Get the reaction solution gained in step (1), add 25% n-hexane accounting for the weight of the reaction solution, stir at a speed of 5 rpm for 20 min, then add ice water accounting for 400% of the weight of the reaction solution, Stir at a speed of 5 rpm until the precipitation stops and then filter, wash the filter cake twice with water accounting for 1 / 2 times the weight of the filter cake, and dry it by centrifugation at a speed of 2825r / min for 70 minutes Ethyl acetate and activated carbon 3 times and 0.06 times the weight of the product obtained, stood at -3°C for 16 hours for recrystallization, and dried the recrystallized product at 55°C to obtain the product.
[0060] After testing, the yield of the product of this example was 68.18%. The target product and impurity content were detected by HPLC and standard substances. After testing, the content of the target prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com