A kind of method for preparing rosuvastatin intermediate
A technology for rosuvastatin and intermediates, applied in the field of pharmaceutical intermediates, can solve the problems of long reaction time, expensive catalyst, low yield, etc., reduce the generation of by-products, have a good prospect for industrial production, and reduce production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
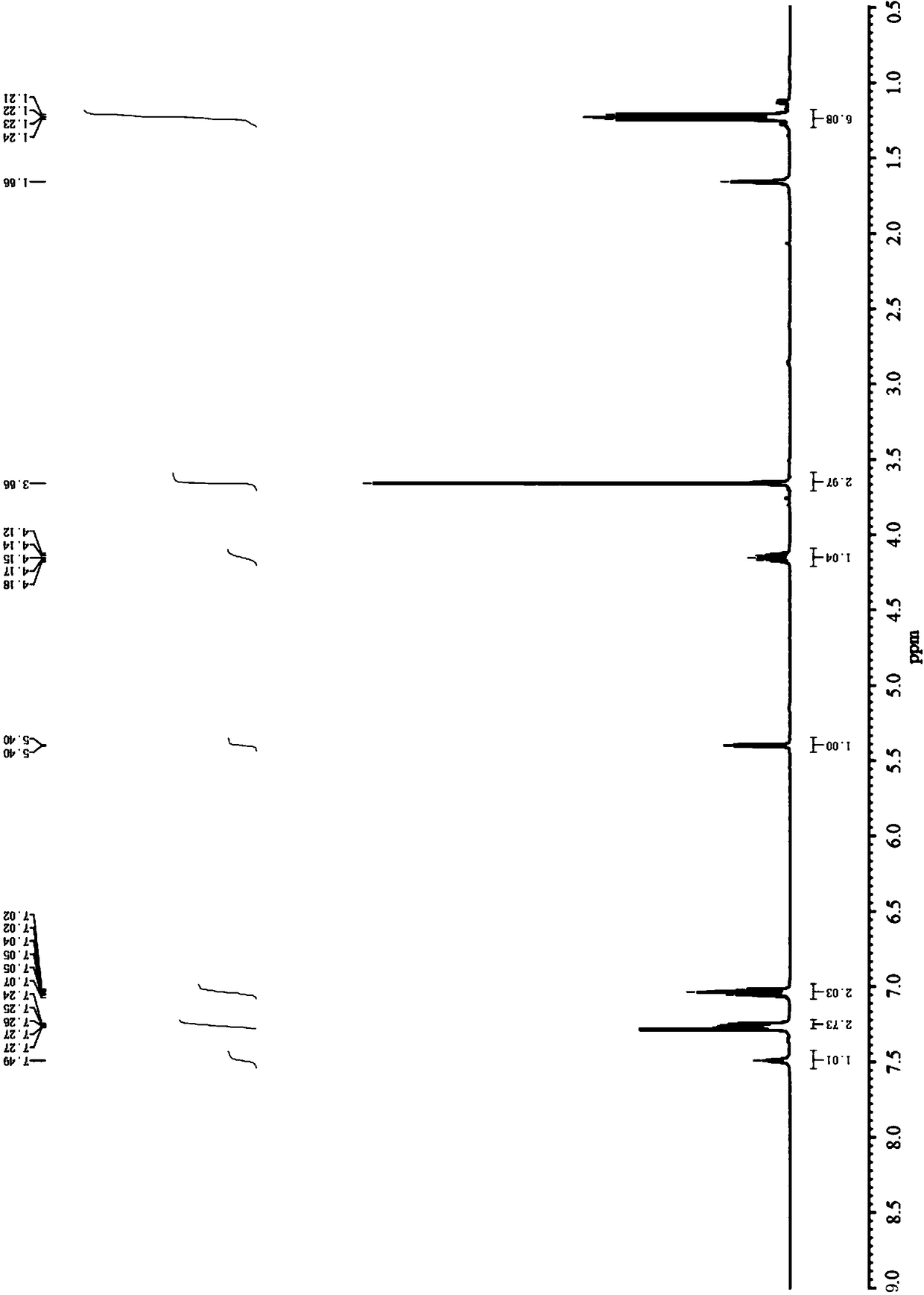
Embodiment 1
[0042] In this example, 4-fluorobenzaldehyde, methyl isobutyryl acetate and thiourea were used as substrates to confirm the activity of related catalysts in catalyzing the bigienlli reaction.
[0043] Add 4-fluorobenzaldehyde (5.0mmol), methyl isobutyryl acetate (6.0mmol), thiourea (7.5mmol) and the corresponding catalyst (0.5mmol) respectively in a three-necked flask equipped with a thermometer and a condenser, and solvent 20mL of 1,4-dioxane, stirred and heated to 90°C, reacted for 12h, the reaction solution was washed with cooling water, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered, distilled under reduced pressure, and then subjected to column chromatography (EA / PE=1:4) isolated and obtained rosuvastatin intermediate 4-(4-fluorophenyl)-6-isopropyl-2-thiocarbonyl-1,4-dihydropyrimidine-5-acetic acid methyl ester.
[0044] Table 1 shows the effect of different catalysts on this reaction
[0045]
[0046]
[0047]
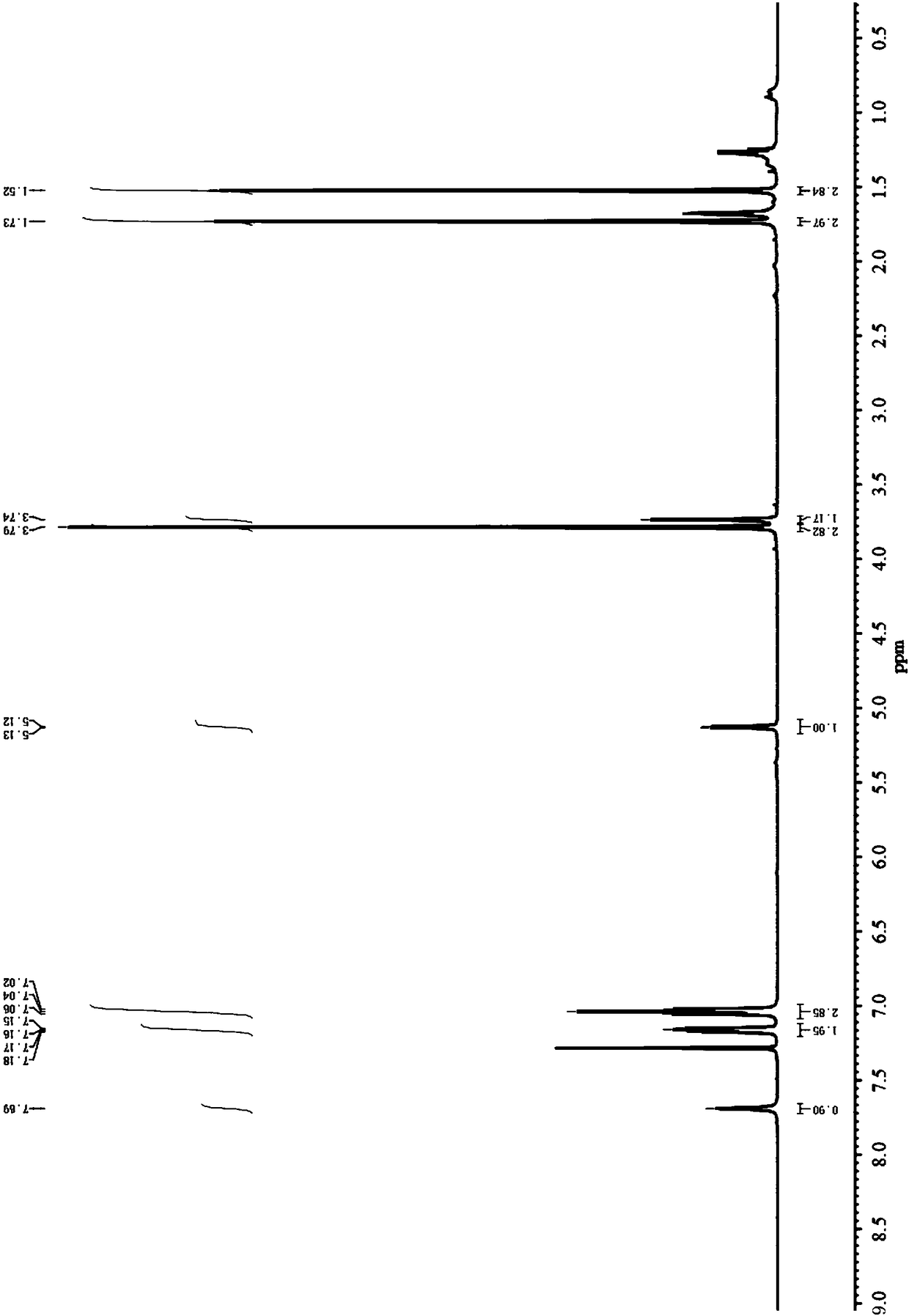
Embodiment 2
[0049] In this example, 4-fluorobenzaldehyde, methyl isobutyryl acetate and thiourea were used as substrates, magnesium chloride was used as a catalyst, and the influence of solvent and temperature on the reactivity was investigated.
[0050] Add 4-fluorobenzaldehyde (5.0mmol), methyl isobutyryl acetate (6.0mmol), thiourea (7.5mmol) and magnesium chloride (0.5mmol) respectively in a three-necked flask equipped with a thermometer and a condenser, and add the corresponding Solvent (20mL), stirred and heated up to the specified temperature, after 12h of reaction, the reaction solution was washed with cooling water, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered, distilled under reduced pressure, and then subjected to column chromatography (EA / PE=1 :4) Separating and obtaining rosuvastatin intermediate 4-(4-fluorophenyl)-6-isopropyl-2-thiocarbonyl-1,4-dihydropyrimidine-5-methyl acetate.
[0051] Table 2 shows the influence of solvent and reaction te...
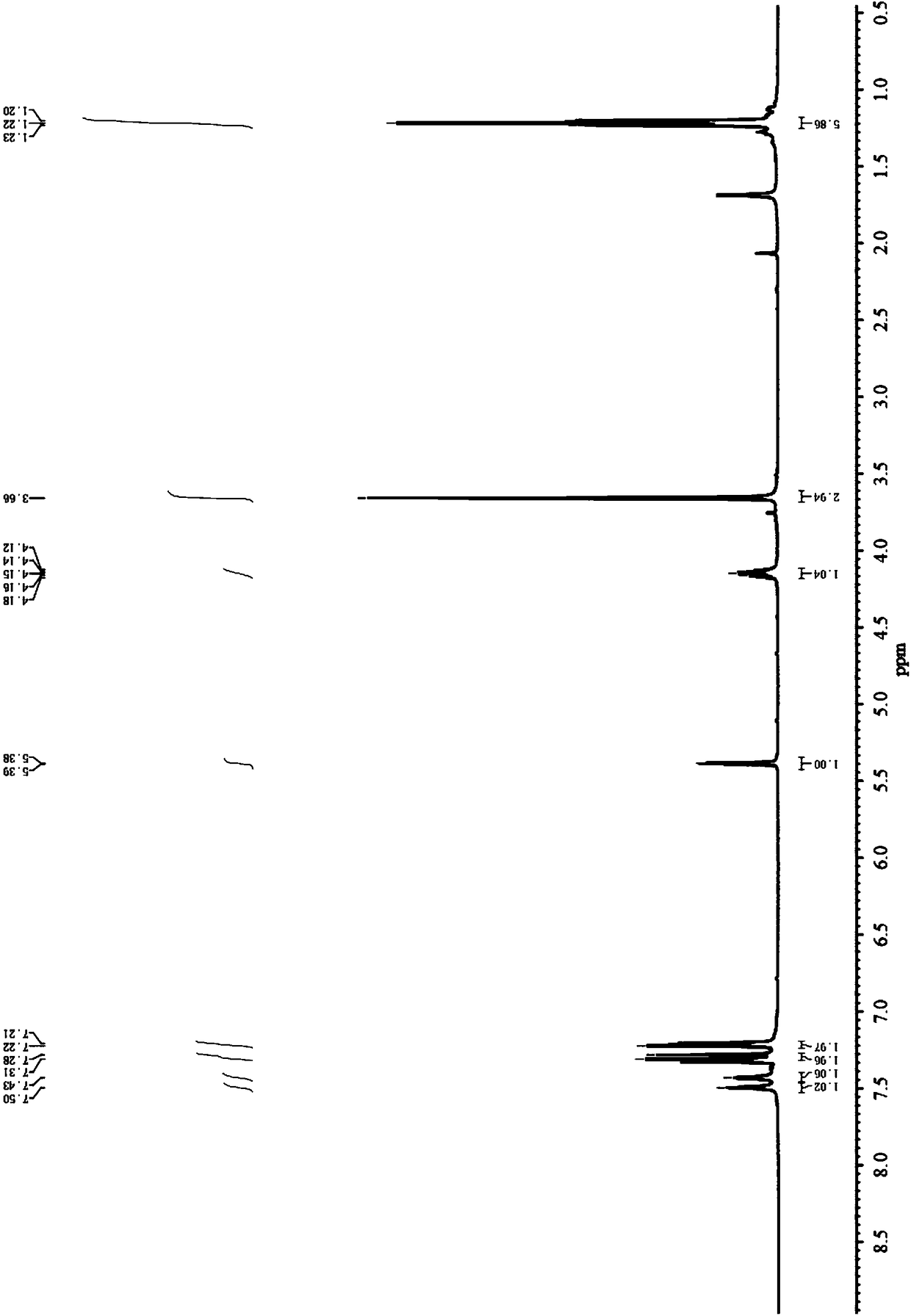
Embodiment 3
[0056] In this example, aromatic aldehyde, isobutyryl acetate and thiourea were used as substrates, magnesium chloride was used as a catalyst, and the stability of the reaction conditions was investigated by changing the R group of the substrates.
[0057] Add aromatic aldehyde (5.0mmol), isobutyryl acetate (6.0mmol), thiourea (7.5mmol) and magnesium chloride (0.5mmol) respectively into a three-necked flask equipped with a thermometer and a condenser, solvent 1,4-diox Hexacyclic 20mL, stirred and heated up to 90°C, tracked by TLC until the reaction was complete, the reaction solution was washed with cooling water, extracted with ethyl acetate, dried with anhydrous magnesium sulfate, filtered, distilled under reduced pressure, and then subjected to column chromatography (EA / PE = 1:4) to obtain rosuvastatin intermediate 4-(4-fluorophenyl)-6-isopropyl-2-thiocarbonyl-1,4-dihydropyrimidine-5-acetic acid methyl ester.
[0058] Table 3 shows the stability of the reaction conditions t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com