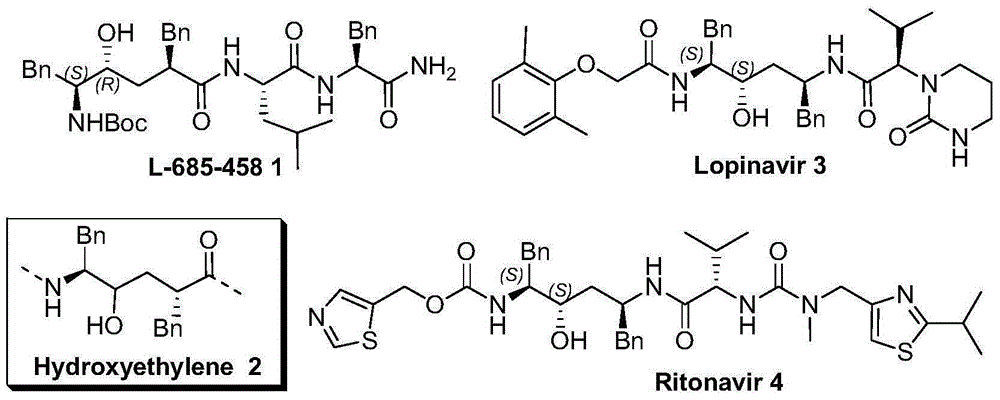
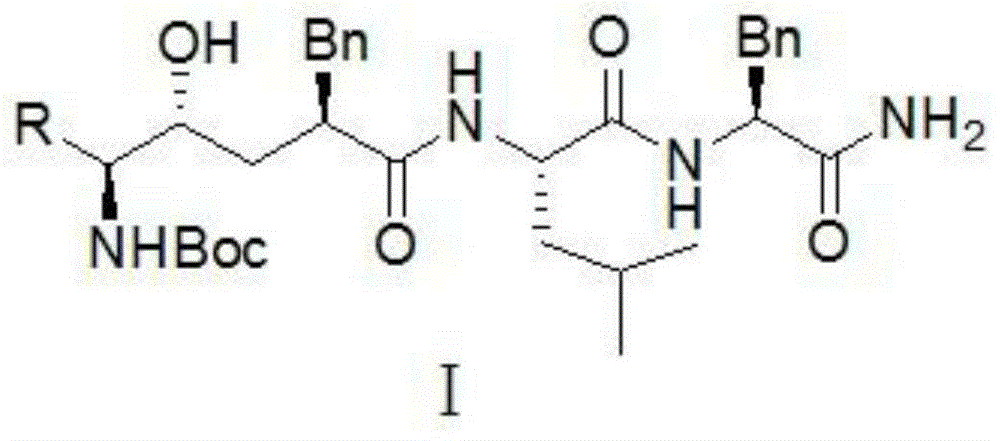
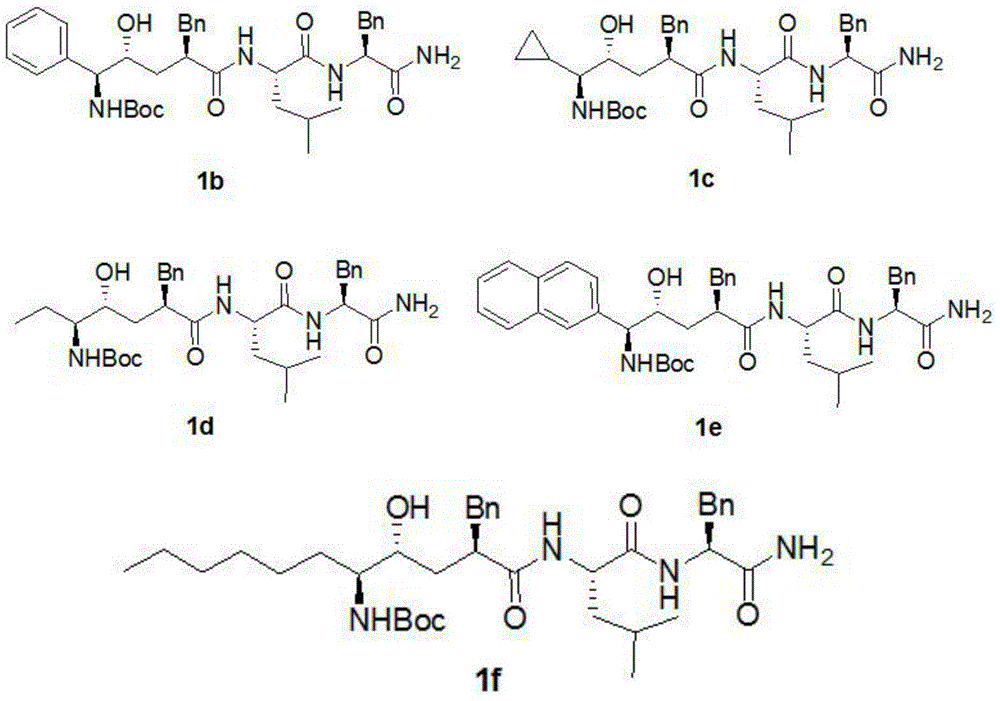
Compound with anti-alzheimer's disease activity and preparation method thereof
A technology for Alzheimer's disease and compounds, which is applied in the field of drug synthesis, can solve the problems of difficult preparation, structural modification of C-5 position-structure-activity relationship research has not been carried out, etc., and achieves the effect of simple process route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of (R)-3-((R)-4,5-bis(tert-butyldimethylsilyloxy)pentanol)-4-phenyloxazolidin-2-one (Compound 2)
[0044] Dissolve compound 1 (10.0g, 26.5mmol) in 120mL tetrahydrofuran and 40mL water, add lithium hydroxide monohydrate (3.4g, 79.6mmol), stir at room temperature for 12 hours, extract with ethyl acetate, concentrate, dissolve in 100mL tetrahydrofuran , cooled to -78°C, added triethylamine (7.3mL, 53mmol), pivaloyl chloride (3.8g, 31.8mmol), oxazolidinone (4.7g, 26.5mmol) and anhydrous lithium chloride (3.4g, 79.6 mmol), reacted at natural temperature for 10 hours, quenched with water, extracted with ethyl acetate, dried, concentrated, and purified by silica gel column to obtain white solid 2 (9.6 g, 69%).
[0045] 1 HNMR (400MHz, CDCl 3 )δ7.38-7.22(m,5H),4.72-4.65(m,1H),4.25-4.15(m,2H),3.84-3.77(m,1H),3.61(dd,J=10.0,5.2Hz, 1H), 3.48(dd, J=10.0, 6.4Hz, 1H), 3.35(dd, J=13.2, 3.2Hz, 1H), 3.14-2.97(m, 2H), 2.77(dd, J=13.2, 9.6Hz ,1H),2.10-2.00(m,1H),1.82-1.72(m,...
Embodiment 2
[0069] The preparation method of compound 1b refers to the preparation method of L-685-4581a.
[0070] tert-Butyl
[0071] (1S,2R,4R)-4-(((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-4-methyl-1-oxopentan-2- yl)carbamoyl)-2-hydroxy-1,5-diphenylpentylcarbamate (1b)
[0072] 1 HNMR (400MHz, CD 3 OD)δ7.32-7.13(m,15H),4.63-4.59(m,1H),4.58-4.47(m,2H),4.22(dd,J=10.0,5.6Hz,1H),3.90-3.80(m ,1H),3.14(dd,J=14.0,6.0Hz,1H),2.90-2.76(m,3H),2.70-2.62(m,1H),1.72-1.56(m,2H),1.54-1.34(m ,11H),1.33-1.25(m,1H),0.92-0.86(m,3H),0.86-0.78(m,3H)ppm.
Embodiment 3
[0074] The preparation method of compound 1c refers to the preparation method of L-685-4581a.
[0075] tert-Butyl
[0076] (1S,2R,4R)-5-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-4-methyl-1-oxopentan-2-ylamino )-4-benzyl-1-cyclopropyl-2-hydroxy-5-oxopentylcarbamate (1c)
[0077] 1 HNMR (400MHz, CD 3 OD)δ7.10-6.96(m,10H),4.43-4.38(m,1H),4.34(dd,J=8.4,6.0Hz,1H),4.00(dd,J=8.6,5.6Hz,1H), 3.50-3.40(m,1H),2.95(dd,J=14.0,6.0Hz,1H),2.74-2.62(m,3H),2.58-2.47(m,2H),1.74-1.55(m,2H), 1.37-1.07(m,13H),0.76-0.65(m,4H),0.64-0.57(m,3H),0.24-0.10(m,2H),0.05--0.04(m,1H),-0.05-- 0.14(m,1H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com