Bovine Morganella morganii arthritis inactivated vaccine and preparation method thereof
A technology of Morganella bovis and Morganella bovis, which is applied in the field of inactivated vaccines for arthritis caused by Morganella bovis and its preparation, can solve the problems of water body and environmental pollution, chemical drug residues, etc., and achieve the goal of preparing The effect of simple process, simple preparation method, and low requirements for vaccine storage and transportation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Isolation and Characterization of Morganella morganii NL-1 (CCTCCNO: M2015583)
[0020] 1. Aseptically take the joint fluid of arthritis-stricken cattle, inoculate it on the common nutrient agar medium by streaking, incubate at 37°C for 24 hours, pick a single colony to obtain a pure culture, and transfer it to the normal nutrient agar slant medium Save for animal regression test and identification. Typical colonies on ordinary nutrient agar plates are round, with a diameter of about 1.5-2.0 mm, colorless or light yellow, translucent, smooth, moist, with neat edges, no water-soluble pigment, and the culture has a special odor.
[0021] 2. Take the pure cultured bacteria on the above-mentioned slant, make a smear specimen, and check the bacterial morphology by Gram staining microscope. Then inoculated in ordinary nutrient medium to expand culture, with 10 9 CFU bacteria were inoculated into the knee joint cavity of two-month-old calves. Gram staining showed that the b...
Embodiment 2
[0025] Preparation and safety testing of Morganella morganii NL-1 (CCTCCNO: M2015583) inactivated vaccine
[0026] 1. Preparation of Morganella morganii NL-1 (CCTCCNO: M2015583) inactivated vaccine
[0027] Streak inoculation of Morganella morganii NL-1 on a common nutrient agar plate, pick a single colony and transfer it to the fermentation medium for expansion culture, as the seed solution. The seed liquid is inoculated into the fermentation medium according to the inoculum amount of 0.1% (V / V), and cultivated at 180 rpm at 37°C for 24 hours, which is the cultured vaccine bacterial liquid. Adjust the number of viable bacteria in the vaccine liquid to 10-50 billion CFU / mL, add formaldehyde with a final volume concentration of 0.5%, and inactivate at 4°C for 72 hours. After the vaccine bacteria liquid is inactivated, aluminum hydroxide adjuvant is added in a volume ratio of 1:4, and mixed to prepare an inactivated vaccine for Morganella bovis arthritis.
[0028] 2. Safety te...
Embodiment 3
[0034] Evaluation of immune effect of Morganella morganii NL-1 (CCTCCNO: M2015583) inactivated vaccine
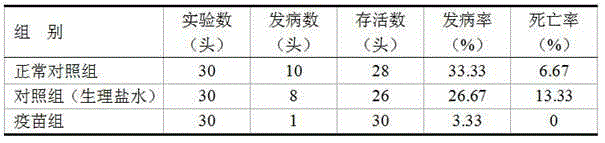
[0035] A total of 90 healthy Holstein bull calves with similar body weight were randomly selected and divided into three groups. Vaccine group: 30 calves were intramuscularly injected with 2 mL of the vaccine of the present invention at the age of 30 days in the neck triangle, and 2 mL of the vaccine of the present invention was injected again at the age of 60 days; Inject 2 mL of sterilized normal saline, and inject 2 mL of sterilized normal saline again at the age of 60 days; normal control group: 30 calves were used as the normal control group without injection. The experimental animals were observed for 6 months, and all experimental animals adopted the same feeding and management method during the observation period. Calculate the morbidity and mortality rate of the experimental animals caused by Morganella morganii during the entire observation period; calculate the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com