LAMP and PCR non-diagnostic detection method for lawsonia intracellularis
A technology for the detection of Lawsonia intracellulare, which is applied in the direction of biochemical equipment and methods, and the measurement/inspection of microorganisms. Effect of short time required and loss reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0032] 1.1 The implementation purpose of this embodiment:
[0033] Aiming at the economic losses caused by the harm of Lawsonia intracellulare to the pig breeding process, the purpose is to provide a simple and effective detection method for rapid diagnosis and detection of Lawsonia intracellulare infection.
[0034] The tissue material containing Lawsonia intracellulare in this example was preserved by the Microbiology and Immunology Laboratory of Sichuan Agricultural University. The tissue material of Lawsonia intracellulare is collected Lawsonia intracellulare-positive porcine ileum mucosal material.
[0035] 1.2.1 Gene sequence amplification of Lawsonia intracellularis suis AM18025.2.1:
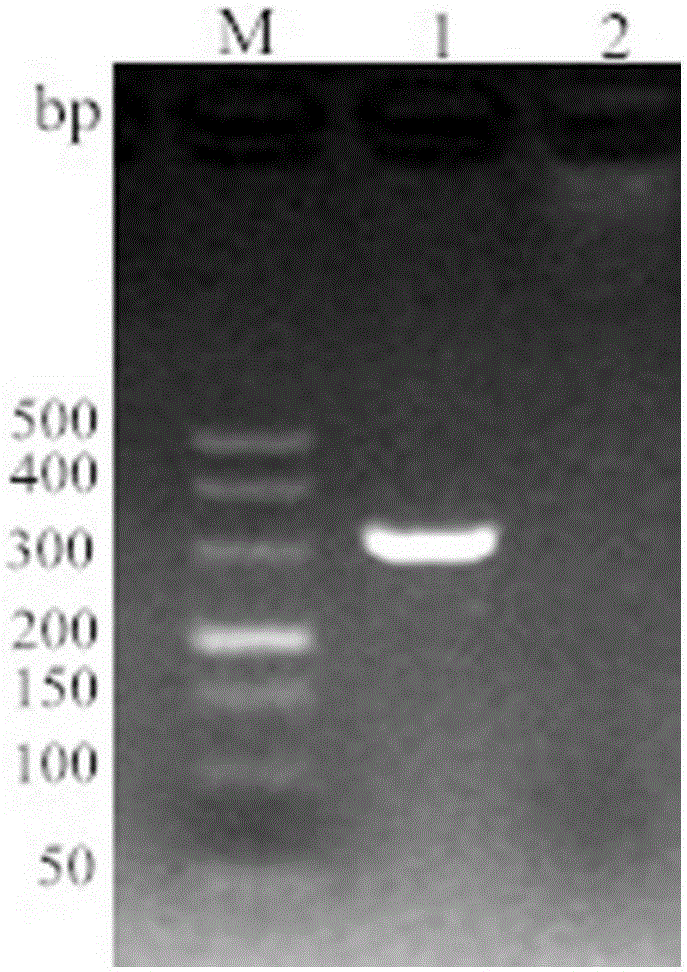
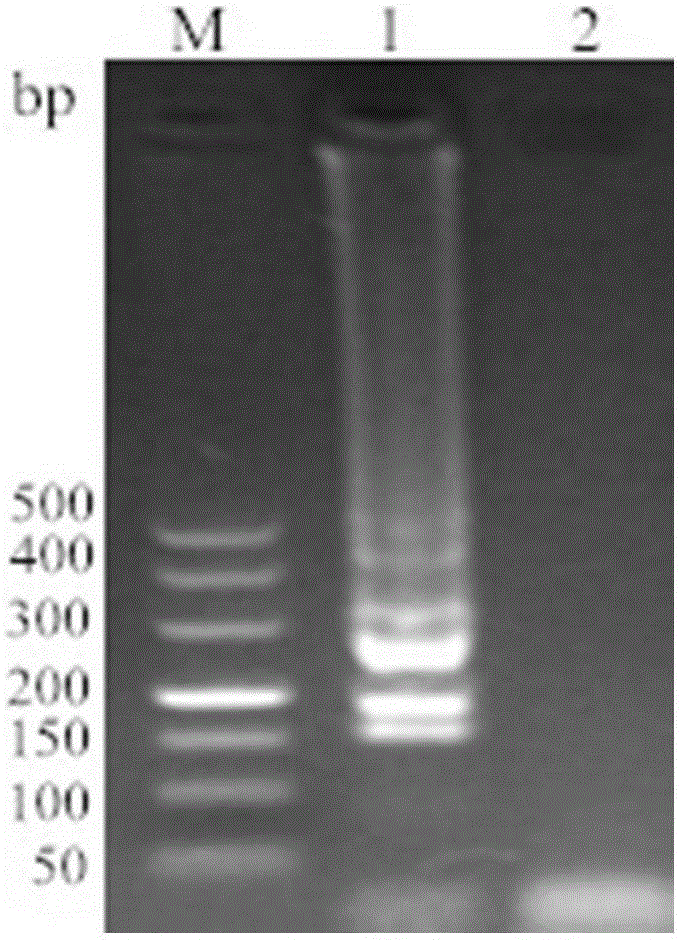
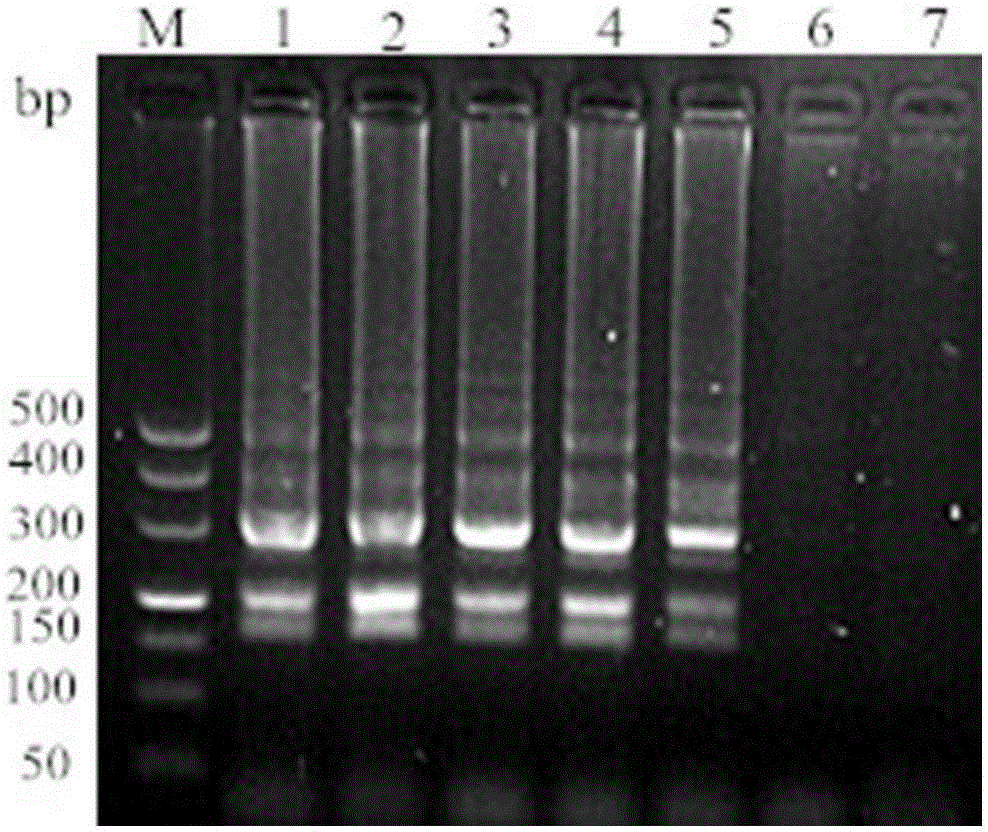
[0036] According to the specific sequence of Lawsonia intracellularis published on Genbank (accession number: gb / AM18025.2.1), a pair of specific primers were designed to amplify a 329bp target fragment. The key factor for the stability of LAMP technology lies in 2 pairs of specific pri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com