Vildagliptin tablet and preparation method thereof
A technology for vildagliptin tablets and tablets, which is applied in the field of pharmaceutical preparations, can solve the problems of increasing material procurement costs, drug production costs, and reducing stability, and achieves the effects of stable quality and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 The preparation of vildagliptin crystal form in the tablet of the present invention
[0026] The vildagliptin crude product is dissolved in n-heptane, the weight of the vildagliptin crude product is 100g, the proportion of the n-heptane is 850mL, then dimethylformamide is added dropwise, when it is found that there is solid precipitation Stop the dropwise addition of dimethylformamide, and let the above reaction solution stand still until an off-white solid is obtained. The off-white solid is filtered and vacuum-dried to obtain a white powder, vildagliptin crystalline form 95.8 g, with a yield of 95.8%.
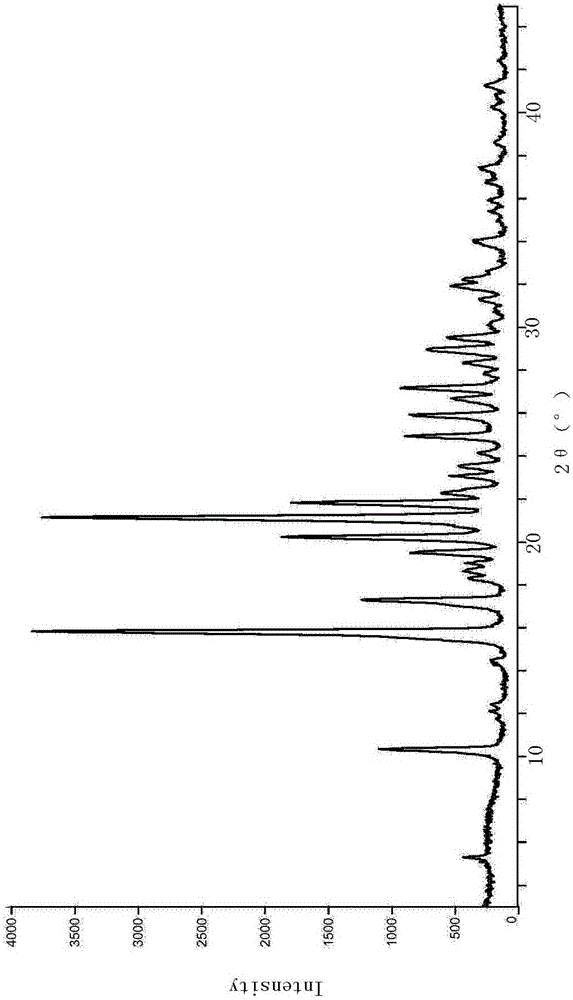
[0027] In the X-ray powder diffraction diagram of this crystal form, the reflection angles 2θ are 5.34, 10.341, 14.438, 15.841, 17.32, 18.301, 18.68, 19.005, 19.577, 20.26, 21.161, 21.859, 22.379, 23.04, 23.5, 24.177, 24.959 There are diffraction peaks at 25.881, 26.641, 27.18, 28.3, 28.999, 29.501, 31.96, 32.258, 33.999, 36.798, 37.38, and 41.297, and the...
Embodiment 2
[0028] Embodiment 2 The preparation of vildagliptin crystal form in the tablet of the present invention
[0029] The vildagliptin crude product is dissolved in n-heptane, the weight of the vildagliptin crude product is 100g, the proportion of the n-heptane is 800mL, then dimethylformamide is added dropwise, when it is found that there is solid precipitation At this time, the dropwise addition of dimethylformamide was stopped, and the above-mentioned reaction solution was allowed to stand still until an off-white solid was obtained. The off-white solid was filtered and vacuum-dried to obtain a white powder, vildagliptin crystal form 94.6g, with a yield of 94.6%.
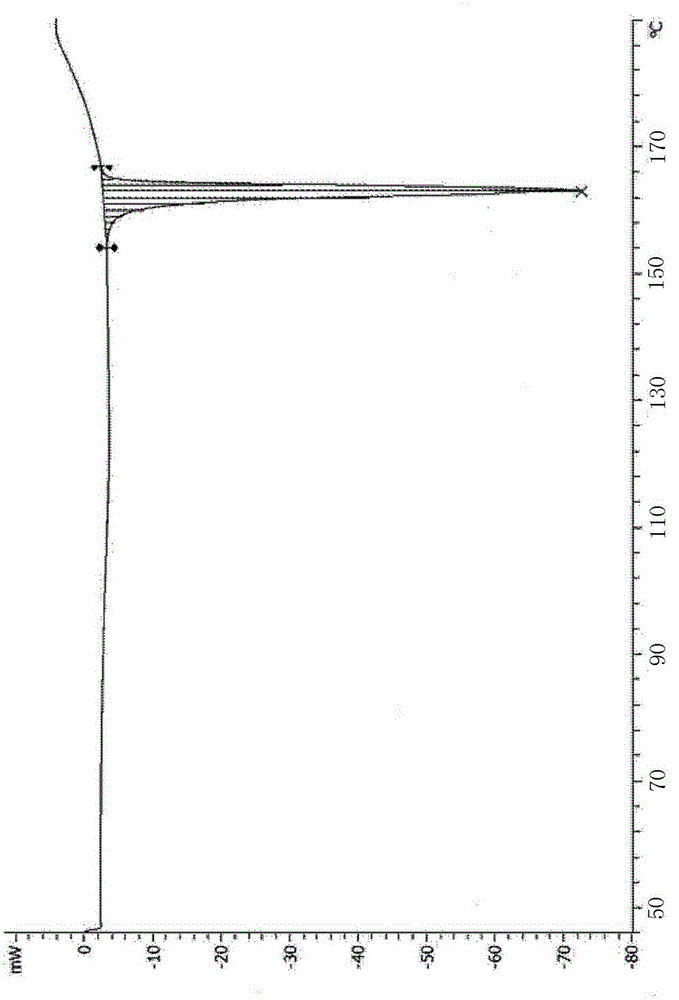
[0030] The X-ray powder diffraction pattern and DSC pattern of the crystal form of vildagliptin are the same as in Example 1.
Embodiment 3
[0031] Embodiment 3 Preparation of vildagliptin crystal form in the tablet of the present invention
[0032] The vildagliptin crude product is dissolved in n-heptane, the weight of the vildagliptin crude product is 100g, the proportion of the n-heptane is 900mL, then dimethylformamide is added dropwise, when it is found that a solid precipitates At this time, the dropwise addition of dimethylformamide was stopped, and the above reaction solution was allowed to stand still until an off-white solid was obtained. The off-white solid was filtered and vacuum-dried to obtain a white powder which was vildagliptin crystal form 93.7g, with a yield of 93.7%.
[0033] The X-ray powder diffraction pattern and DSC pattern of the crystal form of vildagliptin are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com