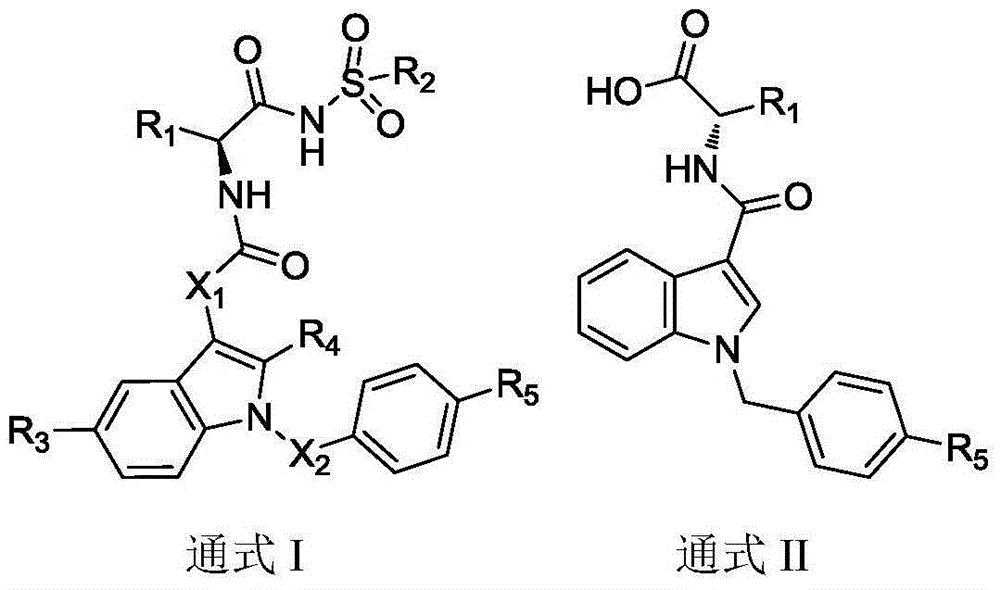
Substituted3-indole Bcl-2 protein inhibitor and preparation method and application thereof
A protein inhibitor, bcl-2 technology, applied in the field of medicine, can solve the problems of tumor cell apoptosis, affect the effect of tumor treatment, and produce drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
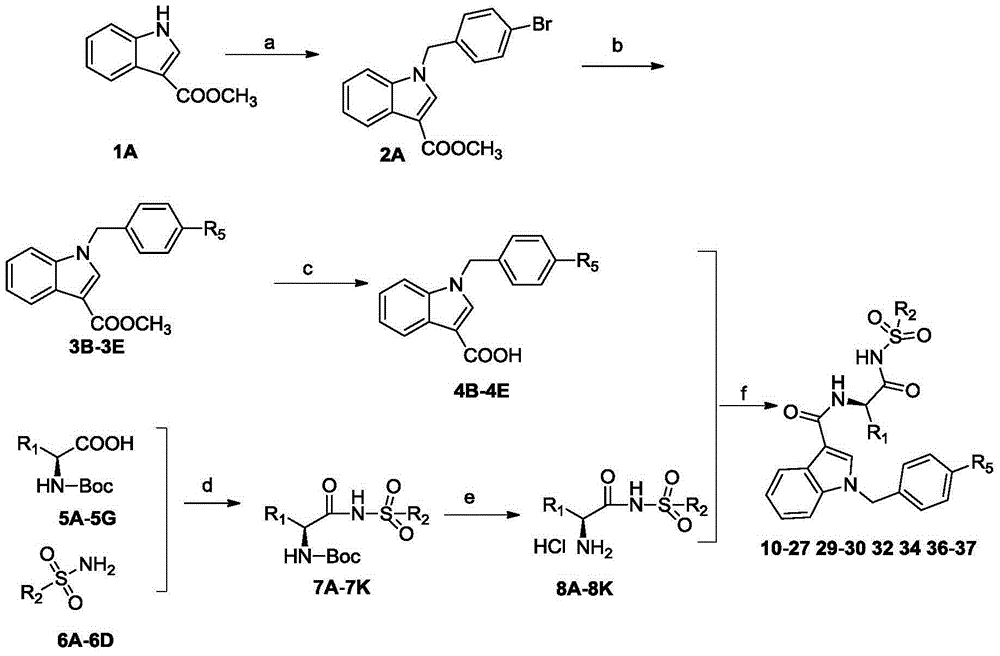
[0084] The preparation method of the substituted 3-indole Bcl-2 inhibitor is one of the following four synthetic routes:
[0085] Synthetic route 1: 3-indole carboxylate methyl ester reacts with p-bromobromobian to obtain intermediate 2A, 2A reacts with various substituted phenylboronic acids to obtain intermediate 3B-3E, and then 3B-3E is desorbed under alkaline conditions. Methyl esters to obtain intermediates 4B-4E, various substituted amino acids 5A-5G, and various substituted benzenesulfonamides through amide condensation reactions to obtain intermediates 7A-7K, then de-Boc to obtain 8A-8K, and finally the important intermediate 4B The target products 10-27, 29-30, 32, 34, 36-37 were obtained by amide condensation reaction between -4E and 8A-8K.
[0086] Synthetic route one:
[0087]
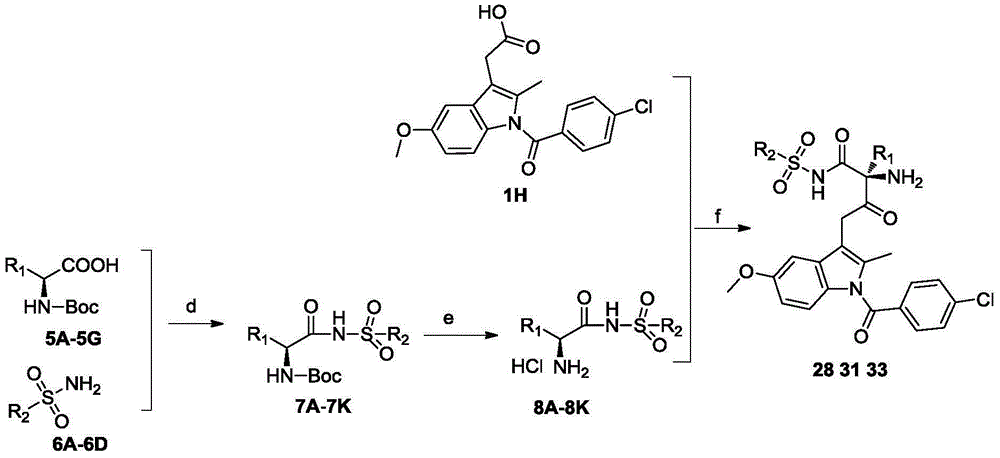
[0088] Synthetic route 2: various substituted amino acids 5A-5G, and various substituted benzenesulfonamides 6A-6D through amide condensation reaction to obtain intermediates 7A-7K, the...
Embodiment 1
[0117] Synthesis of Example 1.1-(4-bromobenzyl)-1H-indole-3-methyl carboxylate (2A)
[0118] Compound 0.7g 1H-indole-3-carboxylic acid methyl ester 1A was dissolved in 20mL DMSO, and 1.1g K 2 CO 3 Stir at 30°C, slowly drop 1.88g of p-bromobromide in DMSO solution, and react overnight. After the reaction, 5 times the volume of water was added, followed by extraction with ethyl acetate, drying over magnesium sulfate, concentration, and column chromatography (P / E=30:1) to obtain a white product (1.1 g, 80%). m.p.117-119°C; 1 H-NMR (400MHz, DMSO-d 6 ),δ8.58(s,1H),8.03-8.01(m,1H),7.72-7.41(m,3H),7.24-7.20(m,4H),5.50(s,2H),3.81(s,3H ).
[0119] Synthesis of 1–((4'-chloro–[1,1'-biphenyl]-4-yl)methyl)-1H-indole-3-carboxylic acid methyl ester (3B)
[0120] Put compound 0.69g2A, 0.004g palladium acetate, 0.016g triphenylphosphine, 0.25g sodium carbonate, 0.33g p-chlorophenylboronic acid into a two-necked flask, add 25mLDMSO, and use N 2 Stir at 80°C after protecting the vacuum. A...
Embodiment 2
[0128] Example 2. (S)-N-((4-chloro-3-nitrophenyl)sulfonyl)-2-(2-(1-(4-chlorophenylcarbonyl)-5-methoxy- Synthesis of 2-methyl-2-methyl-1H-indol-3-yl)acetyl)-3-(4-(4-nitrobenzyloxy)phenyl)propanamide (33)
[0129] Dissolve the compound 0.36g4H in 30mL of dichloromethane, add 0.39gDIEA under ice-cooling, add 0.46gHATU after 10 minutes, turn to room temperature and stir, add 0.46g8F after half an hour, stir overnight, after the reaction is complete, spin off the dichloromethane, Extracted with ethyl acetate, washed with citric acid, dried over anhydrous magnesium sulfate, concentrated and column chromatography (P / E=1:1) gave a light yellow solid (0.25g, 23%), m.p.122-124°C; 1 H-NMR (400MHz, DMSO-d 6 ), δ12.83(s, 1H), 8.53(d, J=2.0Hz, 1H), 8.34(d, J=6.8Hz, 1H), 8.26(d, J=6.8Hz, 1H), 8.13(dd ,J 1 =8.8Hz,J 2=2.0Hz,2H),7.80(d,J=8.4Hz),7.72-7.59(m,5H),7.05(d,J=8.4Hz,1H),6.92(d,J=8.4Hz,1H), 6.76-6.67(m,3H),5.33-5.13(m,2H),4.46-4.41(m,1H),3.90(s,3H),3.53-3.45(m,3H),2.91(dd,J 1 =13....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com