Cyclopropanecarboxamide derivative H crystal form and preparation method thereof
A technology of cyclopropanecarboxamide and derivatives, applied in organic chemical methods, drug combinations, pharmaceutical formulations, etc., can solve the problems of unfavorable hygroscopicity and instability of cyclopropanecarboxamide derivatives, and achieve excellent high temperature stability, The effect of good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of cyclopropanecarboxamide derivative H crystal form
[0034] Weigh 500 mg of cyclopropanecarboxamide derivative raw materials into a container, add 100 mL of ethanol (analytical grade) and N,N-dimethylformamide (analytical grade) 1:2 mixed solvent, suspend at 35°C for 48 hours, filter , After vacuum drying, a white powder is obtained. The yield was calculated to be 56% by weighing.
Embodiment 2
[0035] Example 2. Characterization of cyclopropanecarboxamide derivative H crystal form by XRPD pattern
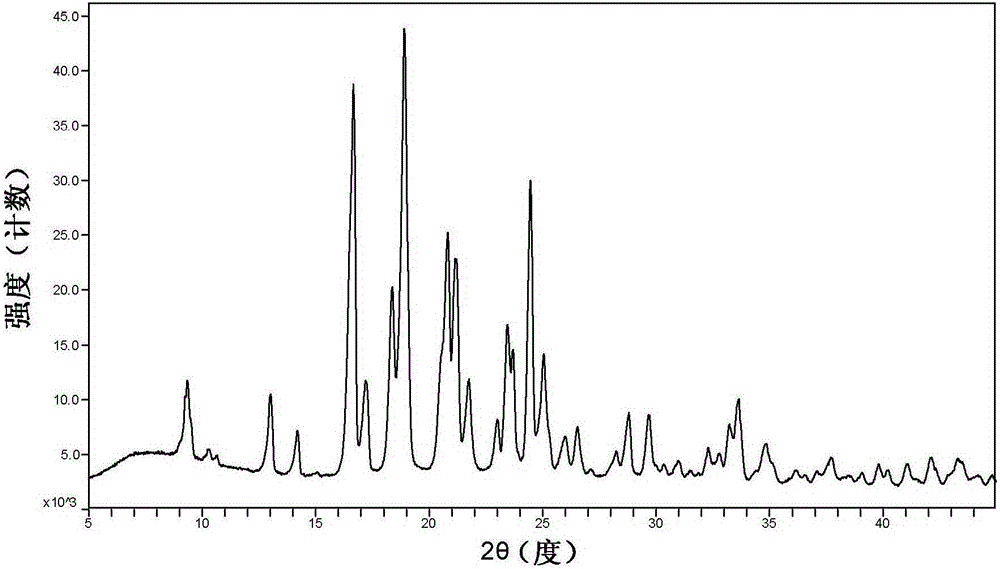
[0036] The measurement of X-ray powder diffraction (XRPD) pattern was carried out with RigakuUltimaIV model combined multi-function X-ray diffractometer. The specific information collected is as follows: Cu anode (40kV, 40mA), scanning speed 20° / min, scanning range (2θ range) 3~45°, scanning step 0.02, slit width 0.01. Samples were processed by pressing glass slides directly on the test plate. Subsequent XRPD patterns were all measured in a similar way.
[0037] Determine the XRPD pattern of the cyclopropanecarboxamide derivative H crystal form prepared according to the method described in Example 1, at 2θ=9.341, 13.019, 14.2, 16.66, 17.201, 18.37, 18.901, 20.522, 20.82, 21.141, 21.759, 23.001, There are diffraction peaks at 23.441, 24.461, 25.059, 26, 26.539, 28.8, 29.661, 33.657, such as figure 1 shown. The error range of the 2θ value is ±0.2. After testing, the err...
Embodiment 3
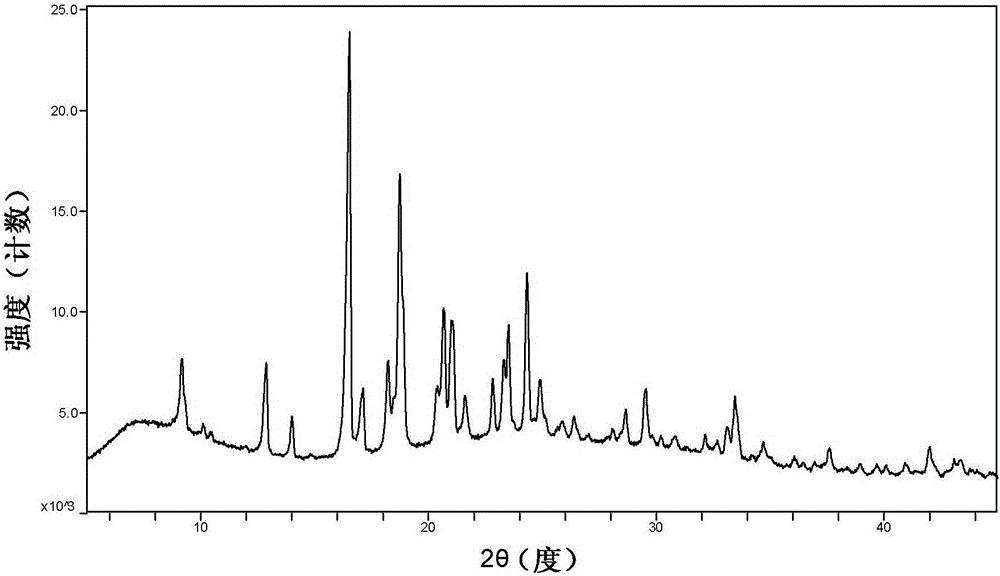
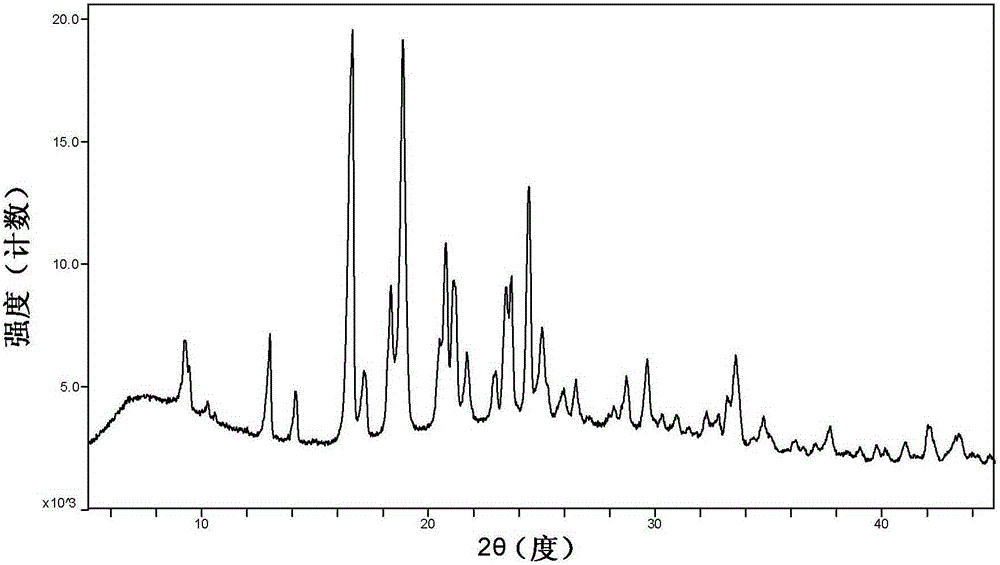
[0039] Example 3. Investigation on the high temperature stability of cyclopropanecarboxamide derivative H crystal form
[0040] The samples of cyclopropanecarboxamide derivative H crystal form were placed in an oven at 60°C, and the samples were taken out for XRPD testing after 5 days and 10 days (such as figure 2 and Figure 5 ) to investigate the crystal stability of the samples against temperature. The results show that the sample of crystal form H is stable at high temperature.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com