Immune toxicity evaluation method for new medicine development
A technology of immunotoxicity and drugs, applied in the field of biomedicine, can solve the problems of no immunotoxicity prediction and safety evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] [Example 1] Separation and preparation of peripheral blood mononuclear cells
[0036] Human peripheral blood mononuclear cells were separated from plasma samples by density gradient centrifugation using Ficoll-Paque (ρ=1.077 g / ml) purchased from GE. The isolated peripheral blood mononuclear cells were treated with RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum and 0.5% blue chain double antibody ( , ThermoFisher) resuspended. About 3-4×10 per 100ml of plasma can be separated 7 Peripheral blood mononuclear cells, the viability of which was detected by trypan blue staining was greater than 95%, and the isolated cells were immediately used for subsequent experimental research.
[0037] 1.1: Exploration of the conditions for the isolation of human peripheral blood mononuclear cells
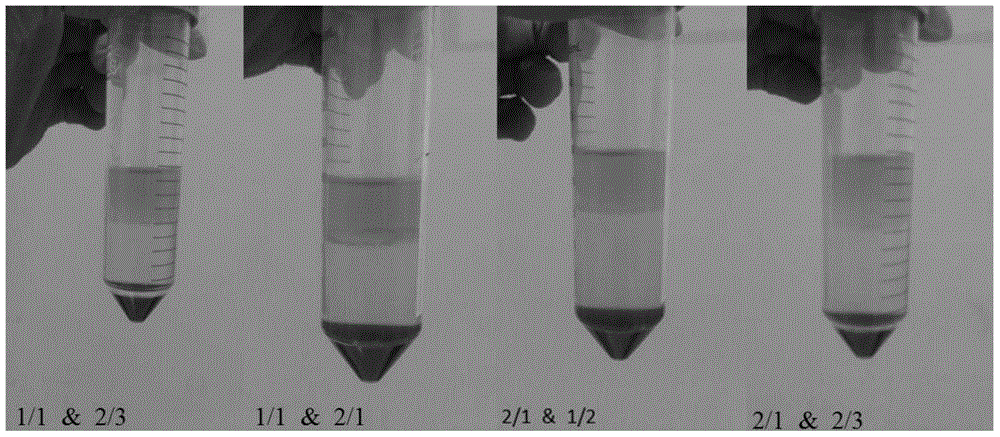
[0038] The key points for separating human peripheral blood are as follows: 1. The dilution degree of plasma; 2. The ratio of plasma to lymphocyte separation medium; T...
Embodiment 2
[0041] [Example 2] T cell proliferation test
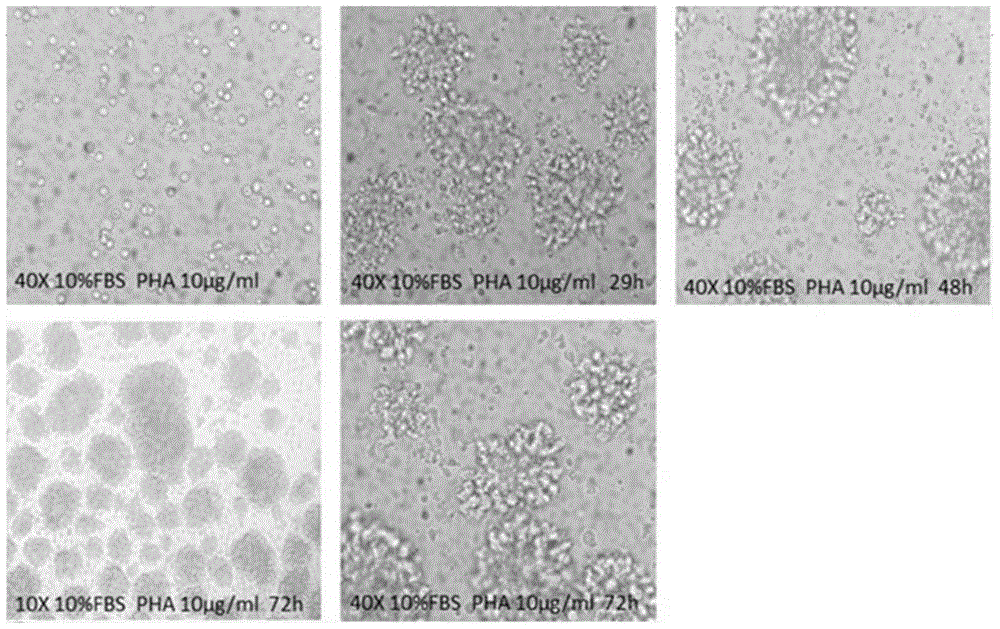
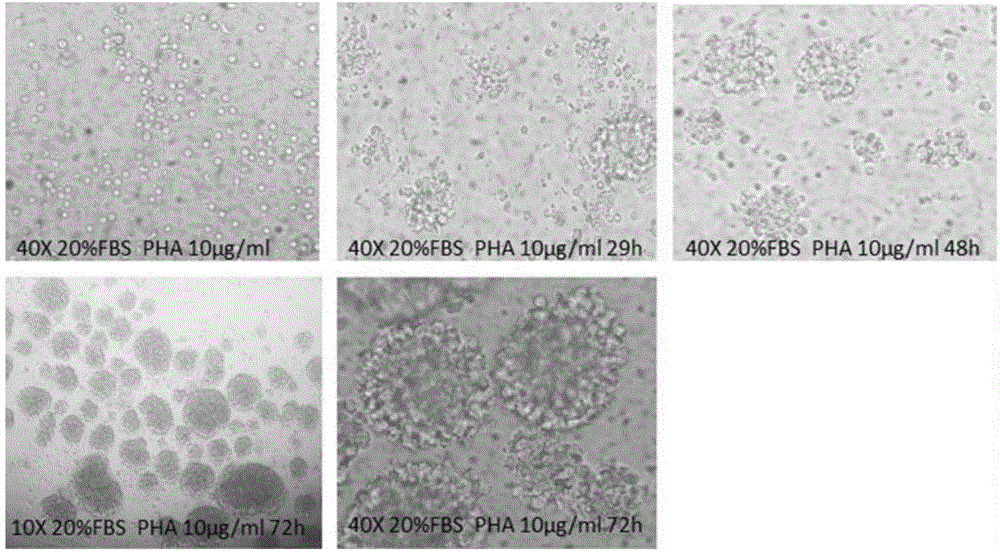
[0042] The proliferation of T cells was detected by CCK-8 method and CFSE staining method respectively. In the detection of CCK-8, human peripheral blood mononuclear cells treated with four test samples (Phytohemagglutinin, PHA, PBS, pHSA, OsrHSA) respectively were seeded in 96-well plates, 100 per well A microliter of medium contains approximately 2 x 10 5 Personal peripheral blood mononuclear cells, the inoculated cells are cultured in a carbon dioxide incubator. After reaching each detection time point (24h, 48h, 72h), add 10 microliters of CCK-8 reagent to each well, and continue to incubate at 37°C for 4h, and then use a microplate reader to detect the absorbance at 450nm. In the CFSE assay, peripheral blood mononuclear cells were stained with 3 μM CFSE at 37°C for 5 minutes and then seeded into 24-well plates. Each well contained approximately 4×10 5 Peripheral blood mononuclear cells, also after reaching each detection t...
Embodiment 3
[0046] [Example 3] Detection of T cell subsets
[0047] The detection of T cell subtypes was all determined by flow cytometry. Peripheral blood mononuclear cells were seeded in 12-well plates after being treated as shown in Table 1, and each well contained about 5×10 5 Peripheral blood mononuclear cells, after reaching each detection time point, the cells were rinsed with PBS and stained with CD3-PE, CD4-FITC and CD8-APC for 30 minutes, and the cells were detected by BD flow cytometry after staining. The ratio of CD3+ / CD4+ and CD3+ / CD8+ cells was detected after lymphocyte circle gate.
[0048] The results showed that the T cells treated with plant-derived recombinant human serum albumin had similar subset ratios to the T cells treated with human serum albumin, as shown in Table 2.
[0049] Table 2. The proportion of subgroups of T cells under different treatments and at different time points
[0050]
[0051]
[0052] a.*p<0.05; **p<0.01. The significant difference in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com