Methods and compositions for generating chondrocyte lineage cells and/or cartilage like tissue
A technique for chondrocytes, cartilage, applied in the field of hypertrophic chondrocytes and growth plate cartilage-like tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0312] result
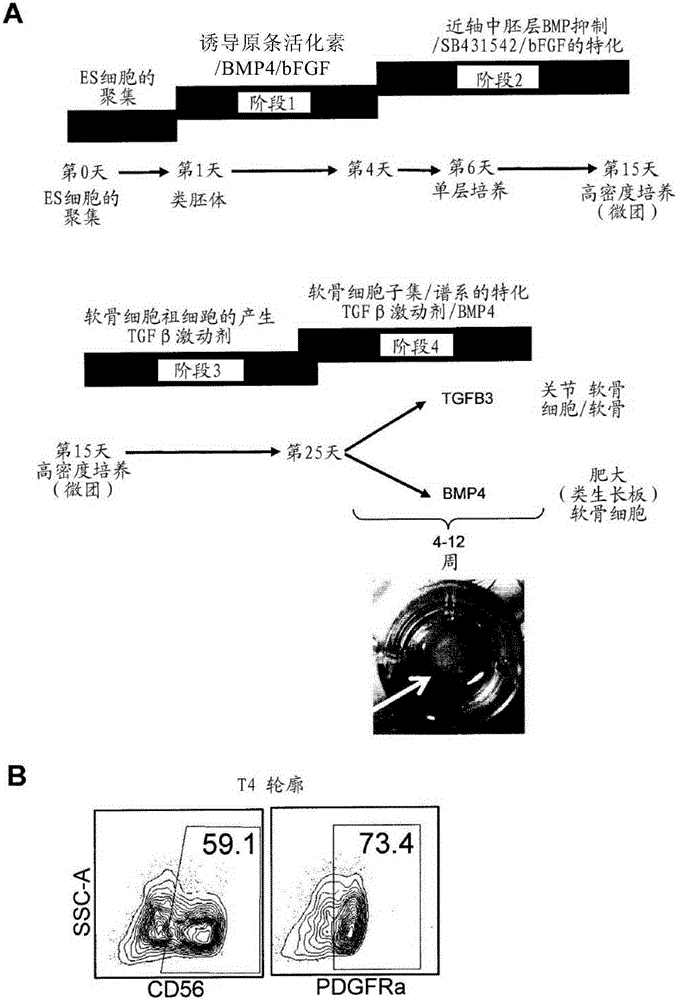
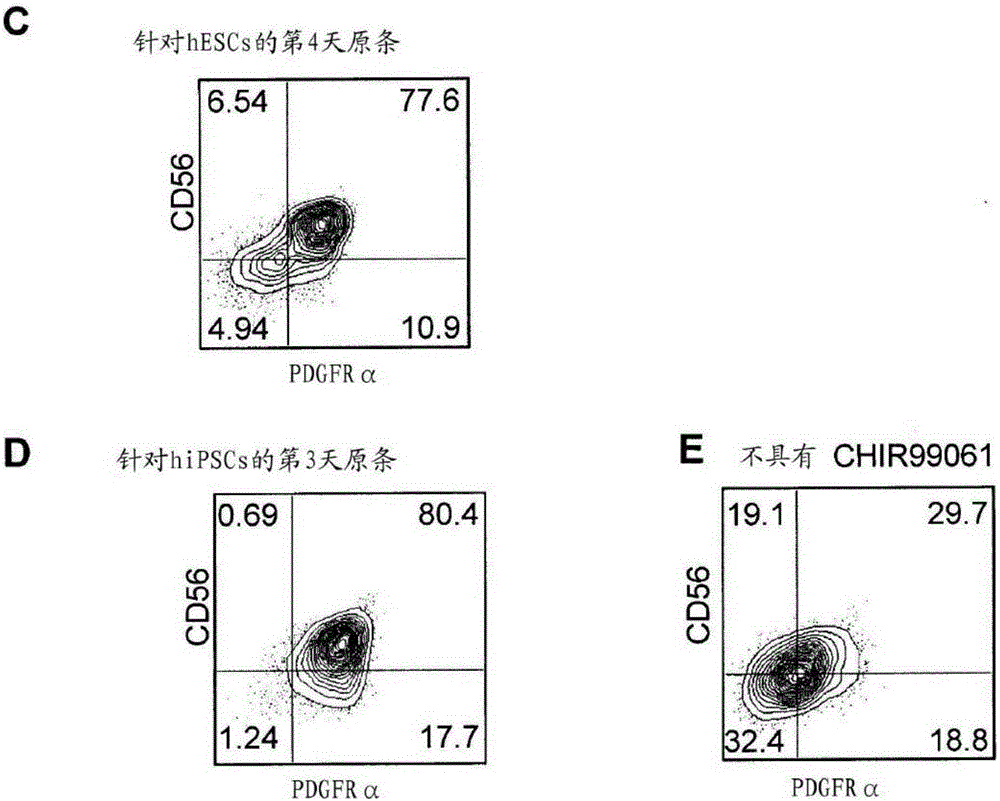
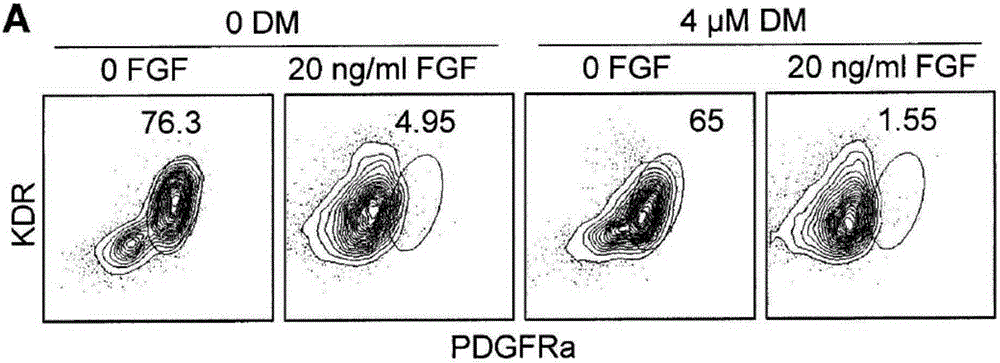
[0313] In vitro formation of chondrocytes including induction of primitive streak (PS) populations into embryoid bodies (stage 1), specification of paraxial mesoderm in monolayer culture (stage 2), and induction of paraxial mesoderm in high-cell-density micellar media Or generation of chondrocyte progenitor cells on collagen-coated membrane filters (Stage 3), and specialized joint and growth plate chondrocytes and cartilage tissue in micelles or filter media (Stage 4) ( figure 1 A).
[0314] The first step in the differentiation from the pluripotent stem cell (PSC) state is the formation of the PS population, which in the embryo occurs during gastrulation upon formation of the three germ layers (endoderm, mesoderm and ectoderm) period. The PS population and endoderm and mesoderm subsets can be induced by PSCs using a combination of activin A (activin, Nodal's surrogate), Wnt, and BMP signaling molecules ((Nostro, Cheng et al. 2008, Kattman, Witty et al.201...
example 2
[0333] CD73+ cells represent joint non-hypertrophic chondrocytes, whereas lack of CD73 positivity allows recognition of growth plate-like hypertrophic chondrocytes.
[0334] Chondrocyte-like and cartilage-like tissues can be produced using the methods described herein, eg, the method described in Example 1. Articular chondrocytes can be isolated and / or compartmentalized from precursor or growth plate-like chondrocytes using the CD73 cell marker. As described herein, when AC-like cells are stimulated with BMP4 they become hypertrophic and lose CD73 expression on their cell surface. One method that can be used to monitor the expression of the CD73 cell surface marker is fluorescence activated cell sorting (FACS) analysis.
example 3
[0336] Use of hESC-derived chondrocytes or cartilage for drug toxicity screening
[0337] HESC-derived chondrocytes obtained using methods described herein, such as in Example 1, can be used for prospective drug toxicology screening and drug discovery. For example, typical cartilage tissue and / or hypertrophic chondrocytes of a growth plate chondrocyte-like cell line and precursors thereof can be contacted with a test substance and assayed for one or more biological endpoints, such as cell death. For example, cell death can be determined using, for example, a viable cell dye exclusion assay, such as the Trypan Blue assay described. For example, AC-like chondrocytes exposed to a test substance (drug) can be monitored for cytotoxicity after a desired time point by counting the cells permeable to the trypan blue dye. Other assays include tetrazolium salt conversion assays. Examples of such assays include the MTT assay and the WST-1 assay. The assay can be automated for high t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com