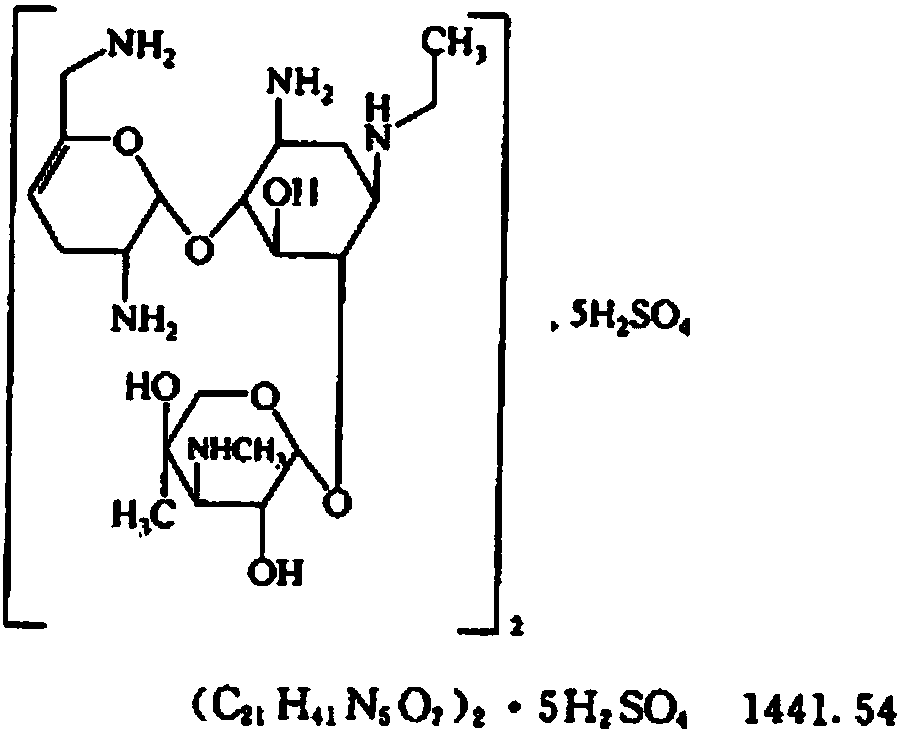
Netilmicin sulfate injection and its quality control method
A technology of netilmicin sulfate and injection, which can be used in pharmaceutical formulations, instruments, antibacterial drugs, etc., can solve problems such as regardless of technical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0235] Embodiment 1: preparation netilmicin sulfate injection
[0236] formula:
[0237] Netilmicin sulfate 50,000 U,
[0238] Anhydrous sodium sulfite 3mg,
[0239] L-cysteine 0.4mg,
[0240] Edetate Disodium 0.2mg, and
[0241] Water for injection, appropriate amount, added to 1ml.
[0242] Preparation method:
[0243] (1) Add cysteine and netilmicin sulfate raw materials to water for injection with a preparation volume of 20% (water temperature below 40°C, the same below), stir to dissolve them, and add 0.2% injection for injection to the liquid medicine Activated carbon, stirred and adsorbed for 30 minutes, filtered;
[0244] (2) Add sulfite and edetate to the water for injection with a preparation amount of 20%, stir to dissolve it, add 0.2% activated carbon for needles to the liquid, stir and absorb for 30 minutes, and filter;
[0245] (3) Mix the medicinal solution obtained in step (1) and step (2), add water for injection of 50% of the preparation amount, mi...
Embodiment 2
[0247] Embodiment 2: preparation netilmicin sulfate injection
[0248] formula:
[0249] Netilmicin sulfate 20,000 U,
[0250] Anhydrous Sodium Sulfite 9mg,
[0251] L-cysteine 0.2mg,
[0252] Edetate Disodium 0.6mg, and
[0253] Water for injection, appropriate amount, added to 1ml.
[0254] Preparation method:
[0255] (1) Add cysteine and netilmicin sulfate raw materials to water for injection with a preparation volume of 20% (water temperature below 40°C, the same below), stir to dissolve them, and add 0.2% injection for injection to the liquid medicine Activated carbon, stirred and adsorbed for 30 minutes, filtered;
[0256] (2) Add sulfite and edetate to the water for injection with a preparation amount of 20%, stir to dissolve it, add 0.2% activated carbon for needles to the liquid, stir and absorb for 30 minutes, and filter;
[0257] (3) Mix the medicinal solution obtained in step (1) and step (2), add water for injection of 50% of the preparation amount, mi...
Embodiment 3
[0259] Embodiment 3: preparation netilmicin sulfate injection
[0260] formula:
[0261] Netilmicin sulfate 100,000 U,
[0262] Anhydrous sodium sulfite 2mg,
[0263] L-cysteine 1mg,
[0264] Edetate Disodium 0.1mg, and
[0265] Water for injection, appropriate amount, added to 1ml.
[0266] Preparation method:
[0267] (1) Add cysteine and netilmicin sulfate raw materials to water for injection with a preparation volume of 20% (water temperature below 40°C, the same below), stir to dissolve them, and add 0.2% injection for injection to the liquid medicine Activated carbon, stirred and adsorbed for 30 minutes, filtered;
[0268] (2) Add sulfite and edetate to the water for injection with a preparation amount of 20%, stir to dissolve it, add 0.2% activated carbon for needles to the liquid, stir and absorb for 30 minutes, and filter;
[0269] (3) Mix the medicinal solution obtained in step (1) and step (2), add 50% water for injection, mix, and optionally use an acid-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com