A kind of pharmaceutical composition for treating diabetes
A composition and a technology for diabetes, applied in the field of medicine, can solve the problems of undiscovered sitagliptin and berberine diabetes, etc., and achieve the effects of improving the therapeutic effect, preventing further deterioration and reducing the side effects of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. KKAy mice (SPF grade, male, 6-8 weeks), provided by Beijing Huafukang Biotechnology Co., Ltd. All experimental animals were used for experiments after adaptive feeding for 10 days.
[0029] 2. Grouping and handling of animals
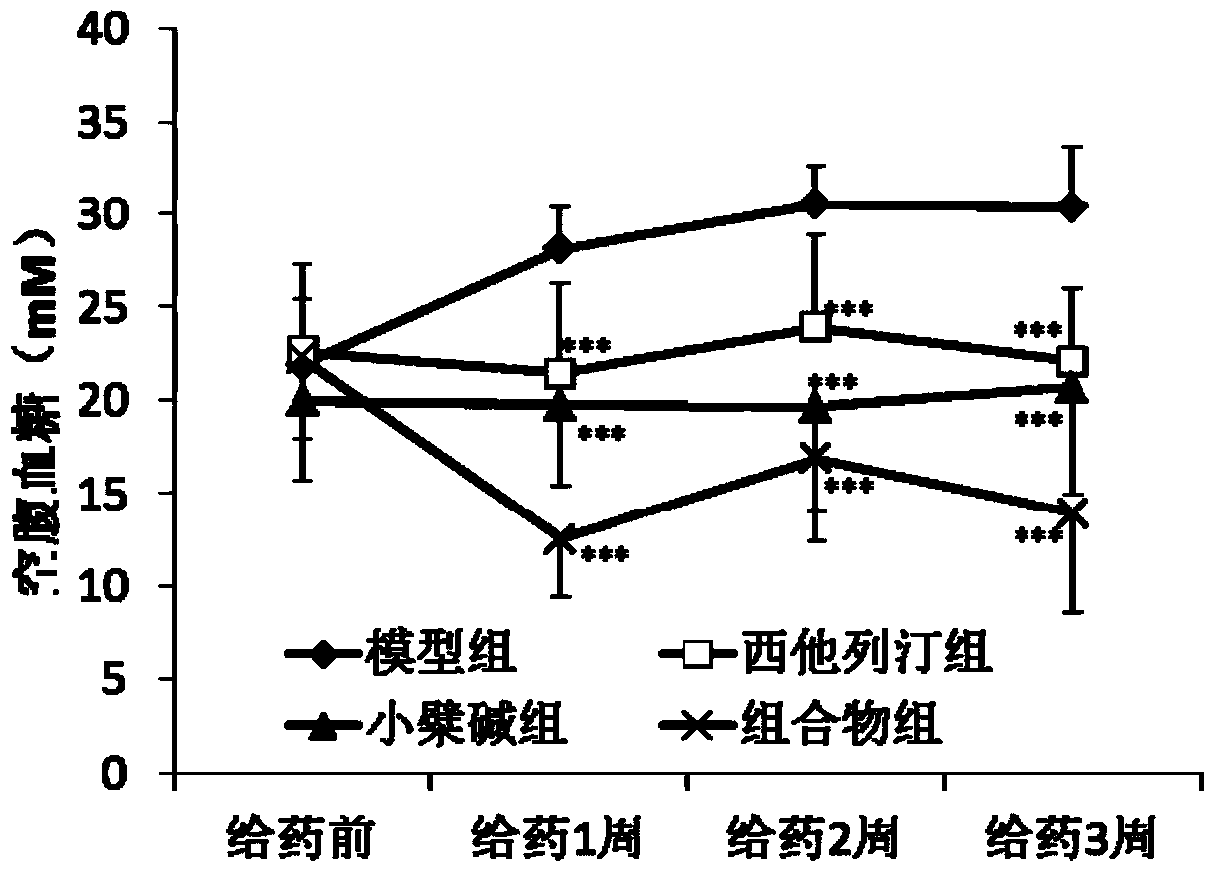
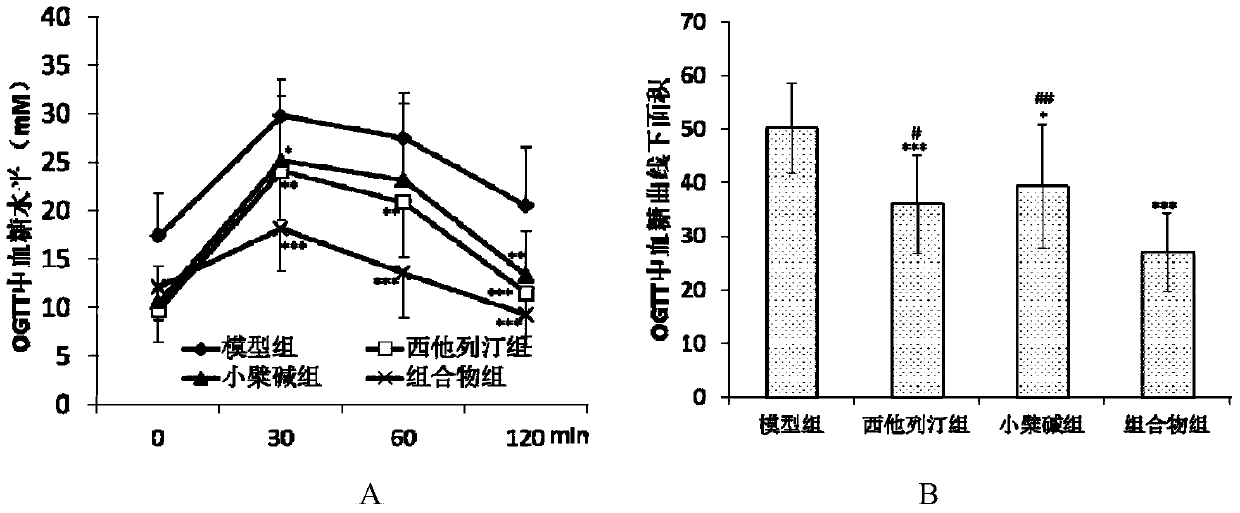
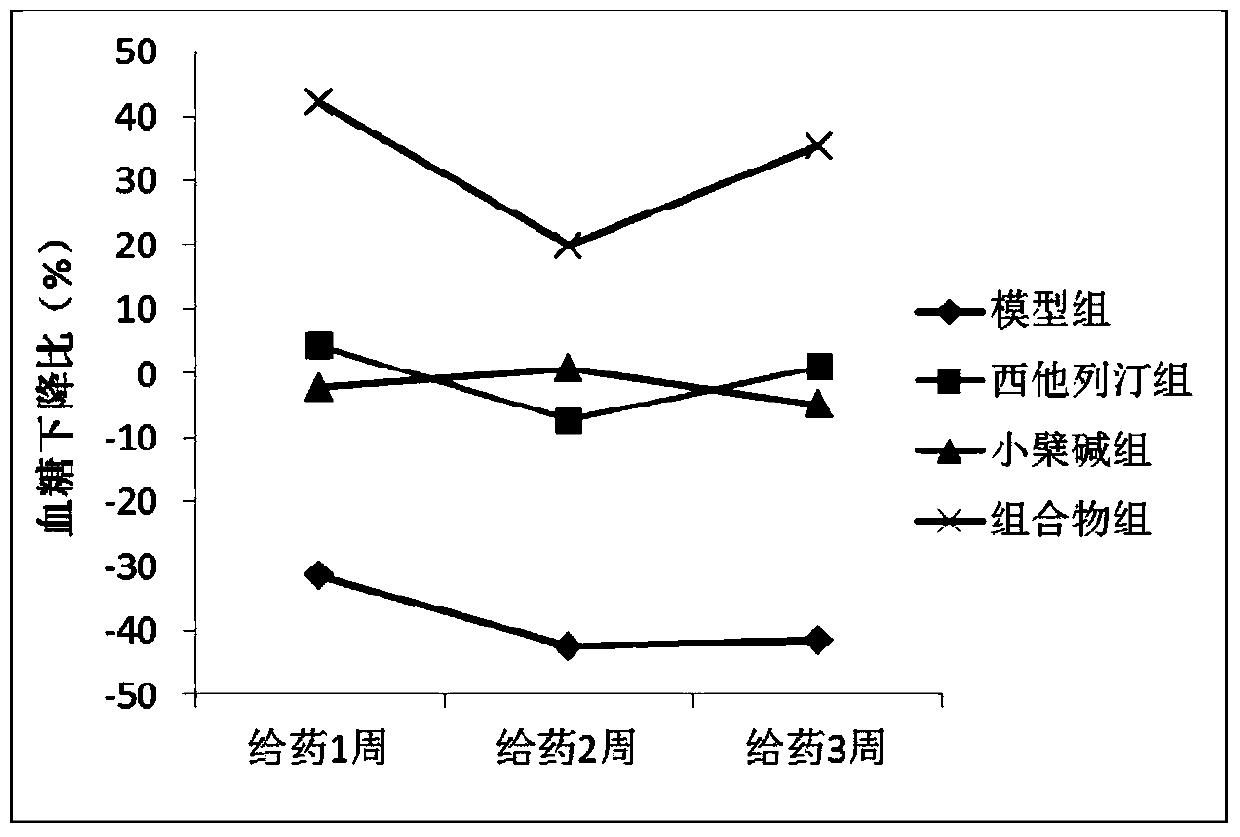
[0030] The hyperglycemic KKAy mice were randomly divided into 4 groups, namely the model group, the sitagliptin group, the berberine group and the sitagliptin and berberine composition group. The grouping and administration are shown in Table 1. All mice were intragastrically administered corresponding doses of drugs every day. The mice in the model group were given the same volume of solvent 0.5% sodium carboxymethylcellulose solution (0.05% CMC-Na).
[0031] Table 1 Animal treatment methods in each group
[0032]
drug
Dose (mg / kg)
model group
\
\
sitagliptin group
sitagliptin
50
Berberine group
300
Composition group
Sitagliptin + Berberine Hydroch...
Embodiment 2
[0043] 1. C57BL / 6, KKAy mice (SPF grade, male), provided by Beijing Huafukang Biotechnology Co., Ltd. All experimental animals were used for experiments after adaptive feeding for about 10 days.
[0044] 2. Grouping and handling of animals
[0045] C57BL / 6 mice were used as a control group, and KKAy mice were randomly divided into two groups: model group, sitagliptin and berberine composition group (sitagliptin 20 mg / kg+berberine hydrochloride 300 mg / kg). All mice were intragastrically administered corresponding doses of drugs every day, and mice in the model group were given the same volume of solvent 0.5% sodium carboxymethylcellulose solution (0.05% CMC-Na).
[0046] 3. Determination of random blood glucose and fasting blood glucose
[0047] After the administration, the random blood glucose of the mice was measured once a week, and the specific operation was as follows: after the administration, blood was collected from the tail vein to detect the glucose level. After t...
Embodiment 3
[0050] Sitagliptin and berberine hydrochloride composition tablet preparation
[0051]
[0052] Preparation process: Pass the microcrystalline cellulose and low-substituted methyl cellulose in the prescription through an 80-mesh sieve, weigh the prescribed amount of sitagliptin, berberine hydrochloride, and mix them evenly with microcrystalline cellulose and low-substituted methyl cellulose , add 2% hydroxypropyl methylcellulose solution to granulate in an appropriate amount, dry, granulate, add prescription amount of magnesium stearate, mix evenly, and compress into tablets to obtain 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com