Hydrochloric acid-promoted efficient system for photocatalytic cyclohexane oxidation of pwv heteropolyacids to ka oil
A technology of cyclohexane and heteropolyacid, which is applied in the field of effective visible light catalytic conversion system, can solve the problems of poor selectivity, weak absorption, and low photocatalytic oxidation efficiency, and achieve the effects of improving absorption efficiency, saving costs, and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-16
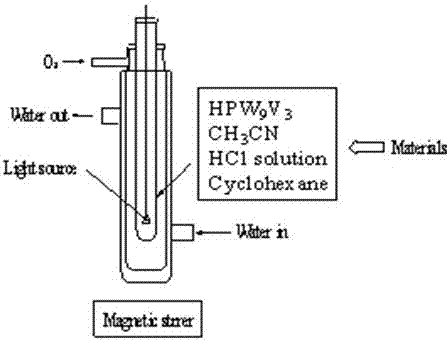
[0022] Examples 1-16: References [Tsigdinos G A and Hallada C, J. Inorg. Chem. 19687 437] Preparation H 4 PW 11 V 1 o 40 (HPW 11 V 1 ), H 5 PW 10 V 2 o 40 (HPW 10 V 2 ) and H 6 PW 9 V 3 o 40 (HPW 9 V 3 ) as a catalyst. Measure 5.0 mL of analytically pure acetocyanide, 1 mmol of analytically pure cyclohexane, 0.010 mmol of homemade PWV heteropolyacid catalyst, and concentrated hydrochloric acid (the concentration in acetonitrile is 0.04-0.3 Mol.L -1 ), put the solution formed by mixing these raw materials into the light source built-in reactor (see figure 1 ), insert a 35-60W halogen tungsten lamp set in the glass tube into the reaction solution, open the oxygen valve to maintain a pure oxygen atmosphere of 1 atmosphere above the reaction solution, and inject 0-5 o C ethanol cooling medium, turn on the magnetic stirring and light and time it. After 4-25 hours of light, the reaction solution was analyzed by Agilent 6890N gas chromatography, the analysis con...
Embodiment 17-28
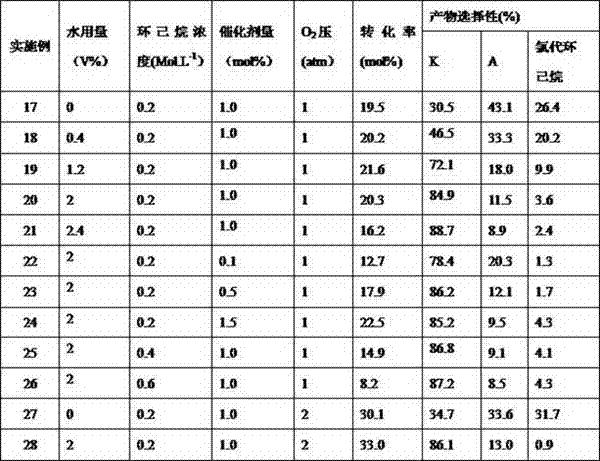
[0024] Example 17-28: Introduce dry HCl gas into 5mL of analytically pure acetonitrile (HCl concentration is 0.18 Mol.L -1 ) Measure 0-0.12mL distilled water, 1-3mmol analytically pure cyclohexane and self-made HPW 9 V 3 Catalyst 0.001-0.015mmol. Then carry out the chromatographic analysis of photocatalytic oxidation reaction and reaction product according to the step described in [0022]. The specific results are shown in Table 2.
[0025] Table 2 Effects of other parameters on the oxidation of cyclohexane by molecular oxygen catalyzed by decatungstate under visible light.
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com