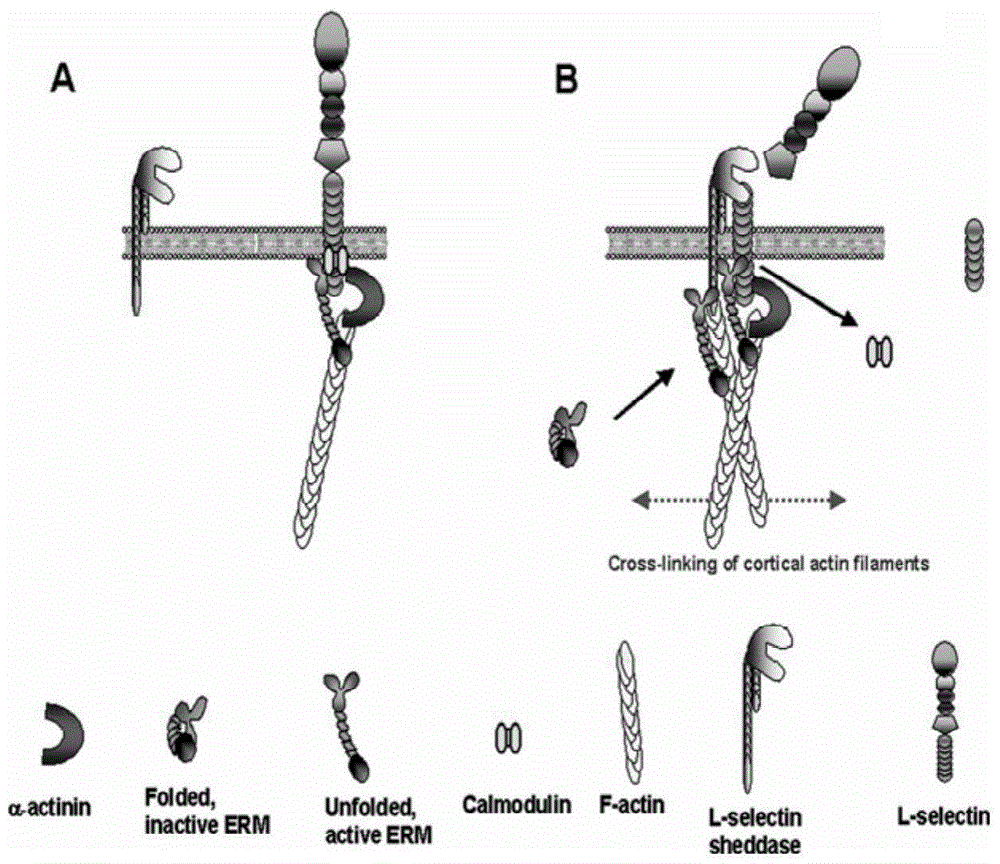
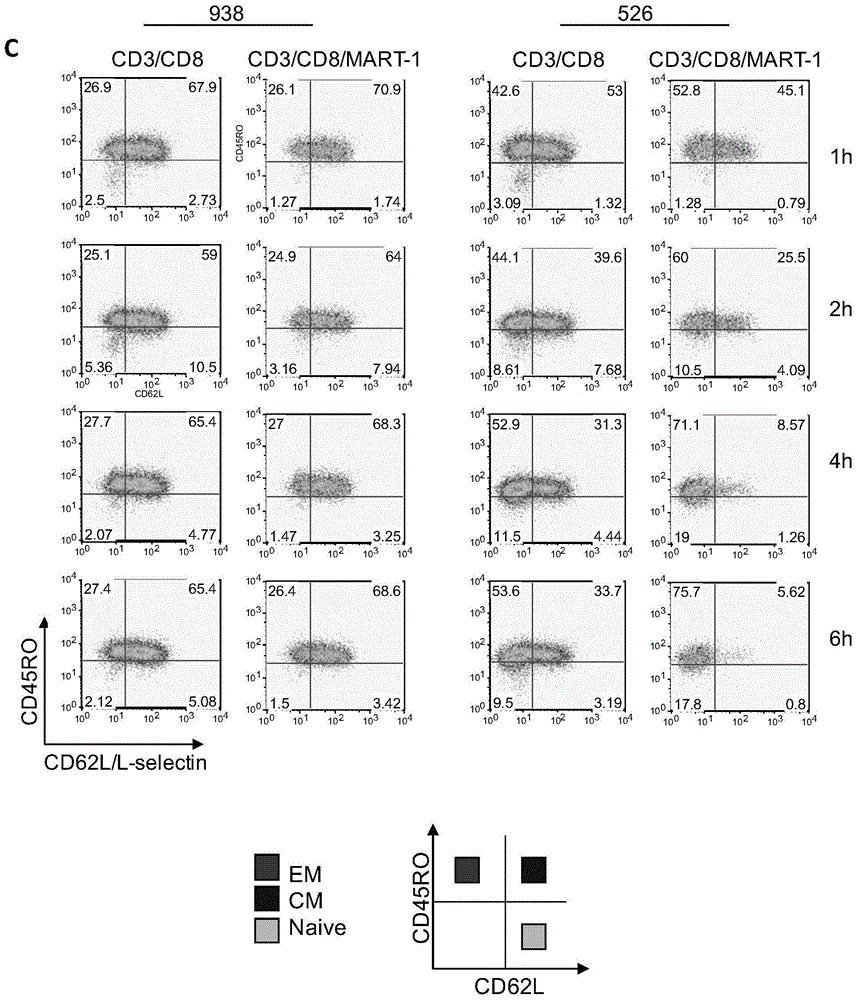
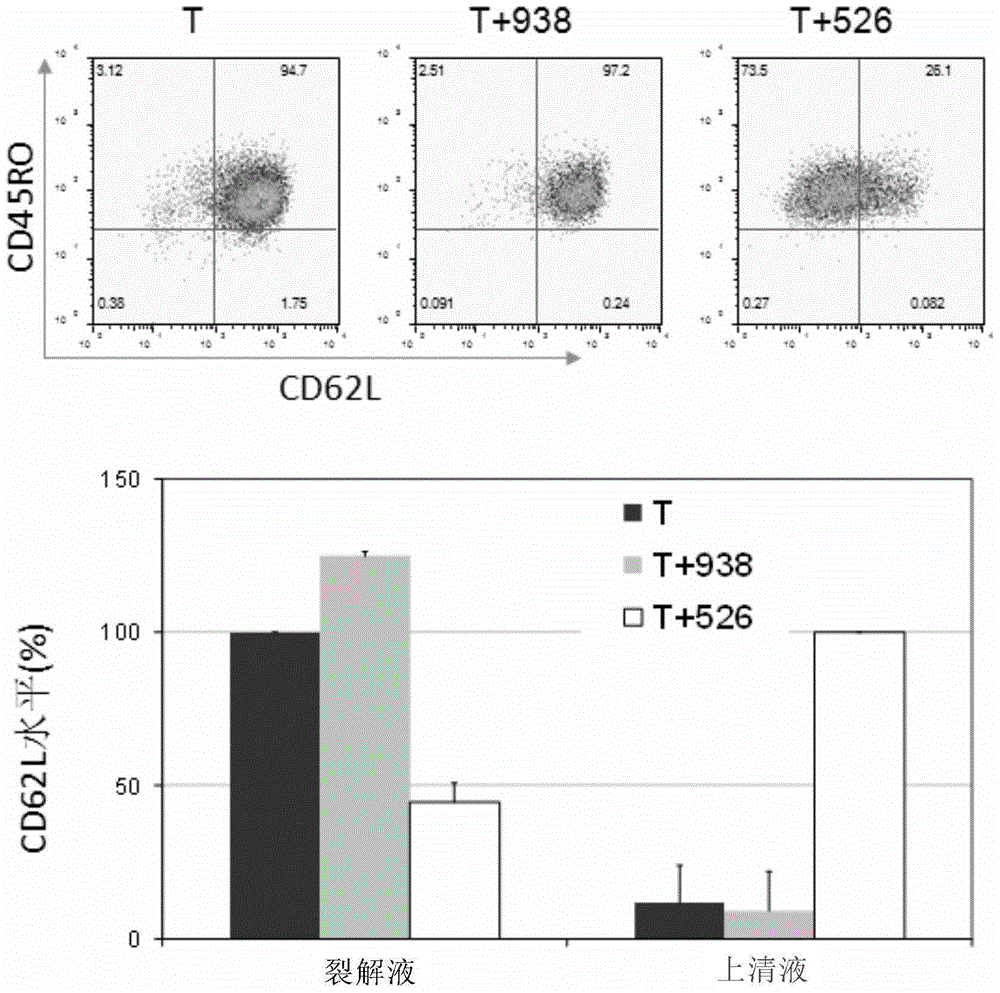
Tumor therapeutic agent improved through IL-12/CD62L fusion protein and preparation method and application thereof
A fusion protein and protein technology, applied in the field of medicine, can solve the problems of IL-12 deregulation, toxic and side effects, and insignificant toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0174] Preparation of fusion protein expressed by lentiviral expression system
[0175] Culture 293T cells. One day before transfection, after adjusting the cell density with DMEM medium containing 10% fetal bovine serum, inoculate 25×10 cells per 15 cm cell culture dish. 6 293T cells at 37°C, 5% CO 2 Cultivate in an incubator, and use for transfection when the cell density grows to 80%-90% after 16h-24h. On the day of transfection, the medium was replaced with a complete medium (DMEM+10% FBS) without antibiotics (P / S). The lentiviral backbones of LVV-MSCV-MART-1TCR, CD62L, hscIL-12L and hscIL-12 / CD62L fusion protein were co-transfected into 293T cells with the other three packaging plasmids, using commercially available calcium phosphate as the medium. After culturing for 6 hours, discard the medium, wash with PBS 3 times and replace with 20 ml of fresh complete medium (DMEM+10%FBS+P / S). Collect the culture supernatant 30-72 hours after transfection, centrifuge at 6000rpm ...
Embodiment 1
[0183] Construction of fusion genes
[0184] The fusion gene was synthesized by Invitrogen Company, and the length and sequence of the fusion gene were confirmed by 1% agarose electrophoresis and sequencing.
[0185] The structure of the obtained fusion gene is shown in SEQ ID NO.:1, and the amino acid sequence of the encoded fusion protein is shown in SEQ ID NO.:2.
Embodiment 2
[0187] Construction of lentiviral expression vector
[0188] Adopt the method described in "Construction of Lentiviral Vector and Gene Modification of T Cells" in the general method: the humanized 293T cells cultured in low-generation DMEM (containing 10% FBS) medium in vitro are counted and transferred to 15CM In the petri dish, the bottom of the petri dish was treated with poly-D-Lysine, and 20X10 6 Cells, on the next day, add a mixed solution of DNA mixed solution and Lipofectamine in each transfection culture dish: the composition of the mixed solution is as follows, get 2mlOptimumI and add pLenti-MSCV (22.5ug), pMD2.G (7.5ug), gag / pol (15ug), pRev (10ug), mix well; at the same time take 2ml OptimumI and add Lipofectamine160ul (Invitrogen), mix well. Mix the two suspensions, incubate at room temperature for 5 minutes, and then drop evenly into the Petri dish. After 48-72 hours, harvest the supernatant containing the genetic engineering vector, centrifuge at 2000g to remo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com