Single domain antibody resisting to human nerve dynein 1 and preparation method of single domain antibody
A ciliary protein and single-domain antibody technology, which is applied in the fields of genetic engineering and antibody engineering, can solve the problems of difficult penetration, affecting the targeting effect, unstable quality of market demand, etc., and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: anti-human neuropilin 1 single domain antibody gene (i.e. heavy chain variable region gene, V H ) clone
[0031] (1) Cells used and mouse-derived anti-human neuropilin 1 monoclonal antibody
[0032] The anti-human neuropilin 1 monoclonal antibody hybridoma cell line was developed by the Anticancer Research Center of Xiamen University School of Medicine. The above cells were routinely cultured in RPMI-1640 medium with 10% fetal bovine serum (Gibco) at 37 °C, 5% CO 2 to cultivate.
[0033] (2) Primer design
[0034] Since the CDR sequence of the variable region gene sequence of the antibody to be amplified is unknown, the primers used in the present invention adopt primer sequences popularized internationally.
[0035] at V H A restriction site NcoI:CATG is added to the 5' end of the primer CCATGG (containing the protective base CATG), at V H A restriction site XhoI: CCG is added to the 3' end of the primer CTCGAG (Contains protective base CCG).
...
Embodiment 2
[0046] Example 2: Construction of expression vector for anti-human neuropilin 1 single domain antibody gene
[0047] Purify V with Gel Purification Recovery Kit (OMEGA) H gene product, then V H The gene product and the pET-22b(+) plasmid (Novagen) were digested with restriction endonucleases NcoI and XhoI (NEB), respectively, and the gel was recovered. Under the catalysis of T4DNA ligase (NEB), the anti-human neuropilin 1 single domain antibody gene (V H Gene) cloned into plasmid pET-22b(+) to construct recombinant plasmid pET-22b(+)-V H , transformed into E.coliBL21(DE3), positive clones were screened by PCR and sent to Shanghai Yingjun Biotechnology Co., Ltd. for sequencing.
Embodiment 3
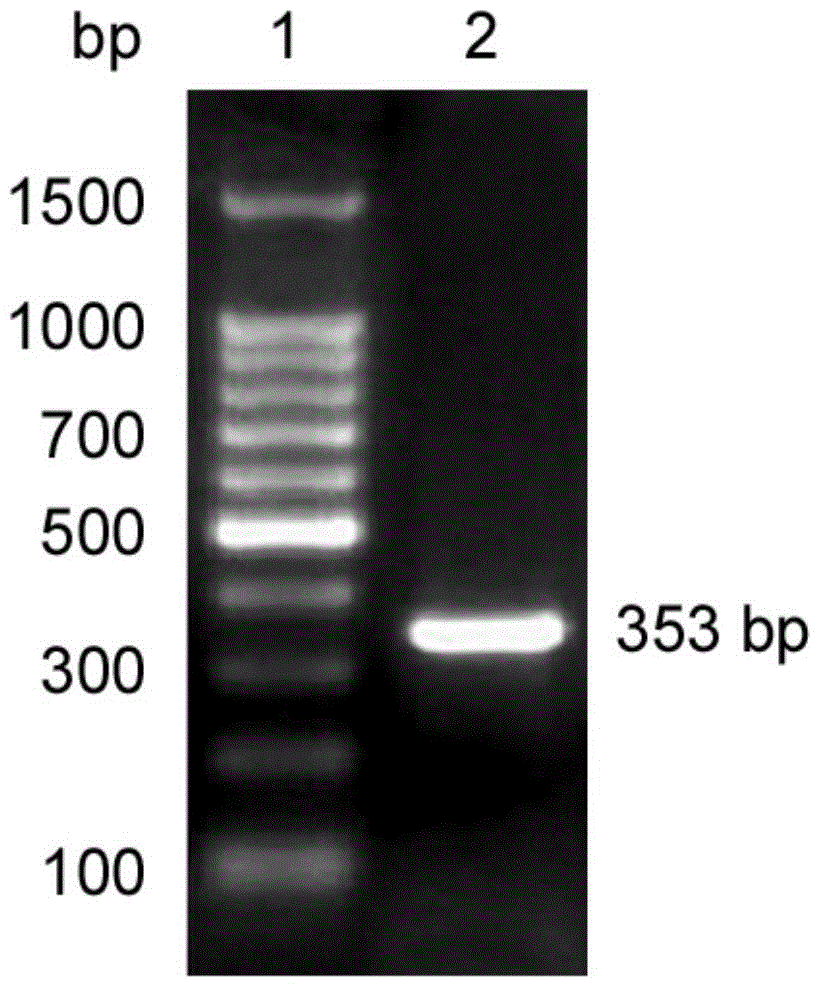
[0048] Example 3: Prokaryotic expression and purification of anti-human neuropilin 1 single domain antibody gene
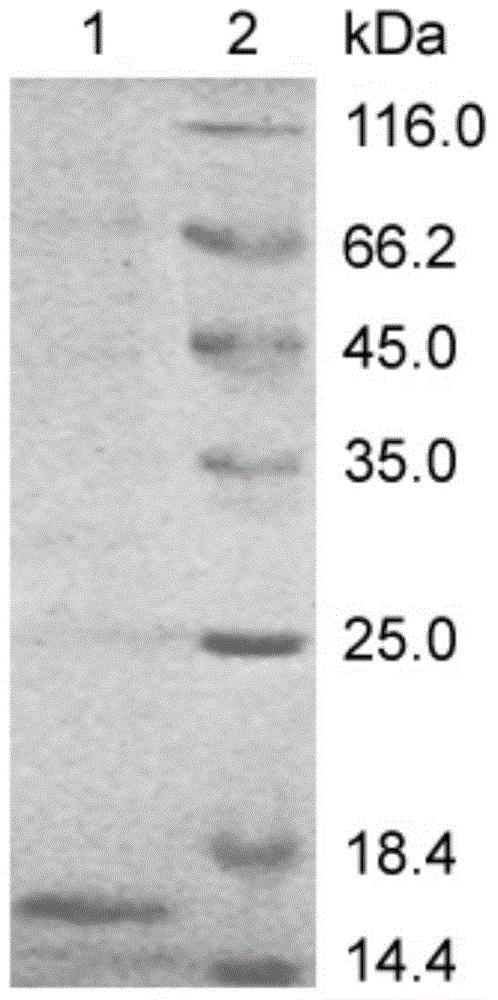
[0049] Select the correct sequencing containing pET-22b(+)-V H Single colony of E.coliBL21(DE3) with recombinant plasmid, cultured with shaking at 37°C overnight, diluted 1:100 into LB culture medium, and cultured with shaking until OD 600nmWhen the value is about 0.6-0.8, add IPTG (Sigma) with a final concentration of 0.5mM to induce expression for 4h. The bacteria were collected for SDS-PAGE electrophoresis. After staining and decolorization, it was found that the expression product of E.coliBL21 (DE3) had an obvious band at 16kDa, which was consistent with the expected target protein band size, indicating that the anti-human neuropilin 1 monoclonal Domain antibody genes are expressed (see figure 2 ).
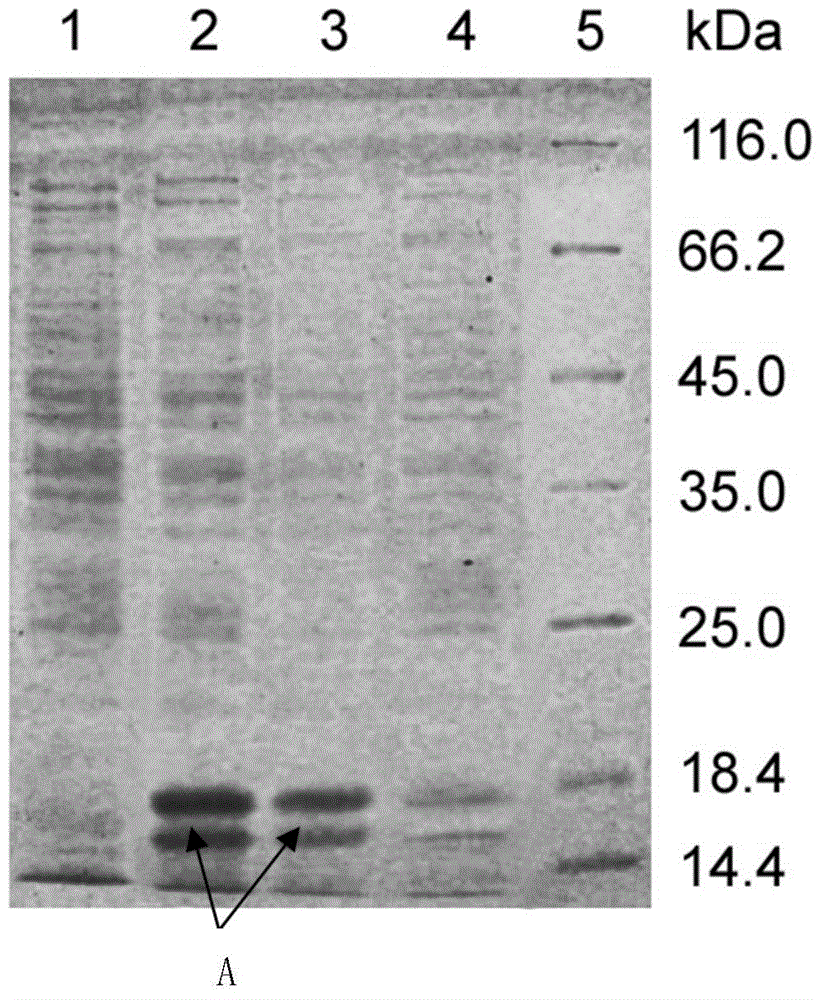
[0050] The results of SDS-PAGE electrophoresis also showed that most of the prokaryotic expression products of the anti-human neuropilin 1 single domain anti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com