Method for detecting ferric ions by using fluorescent carbon quantum dots
A technology of carbon quantum dots and ferric iron, applied in the field of using fluorescent carbon quantum dots to detect ferric ions, can solve the problems of poor selectivity, complicated preparation and processing processes, and the inability to detect Fe alone, and achieve the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] (1) preparing glucose and absolute ethanol mass ratio is 5:1 dispersion liquid;
[0026] (2) The above-mentioned mixed solution was placed in a hydrothermal reaction kettle, and the hydrothermal reaction kettle was placed in an environment of 150° C. to react for 18 hours to obtain fluorescent quantum dots.
[0027] (3) Add 1ml of the above-mentioned carbon quantum dot solution to 8 quartz fluorescence cuvettes respectively;
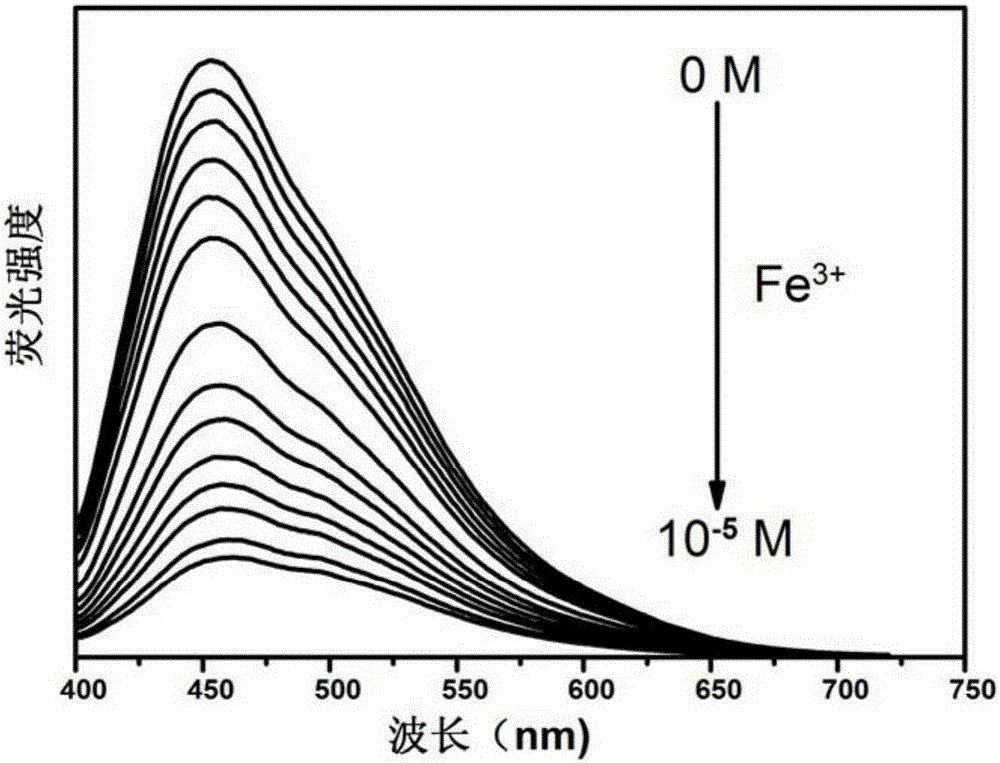
[0028] (4) Use a pipette gun to adjust the concentration to 0; 2×10 -8 ;4×10 -8 ;2×10 -7 ;8×10 -7 ;2×10 -6 ;6×10 -6 ;4×10 -5 The FeCl3 solution joins in the above-mentioned cuvette that carbon quantum dot is housed, and it is put into the fluorescence spectrophotometer;
[0029] (5) After testing, the optimal excitation wavelength of the above-mentioned carbon quantum dots is 360nm, and the excitation wavelength of the fluorescence spectrophotometer is adjusted to the above-mentioned excitation wavelength, and the change curve of the fluore...
example (2
[0036] (1) prepare glucose and absolute ethanol mass ratio 15:1 dispersion liquid, place it in the hydrothermal reaction kettle;
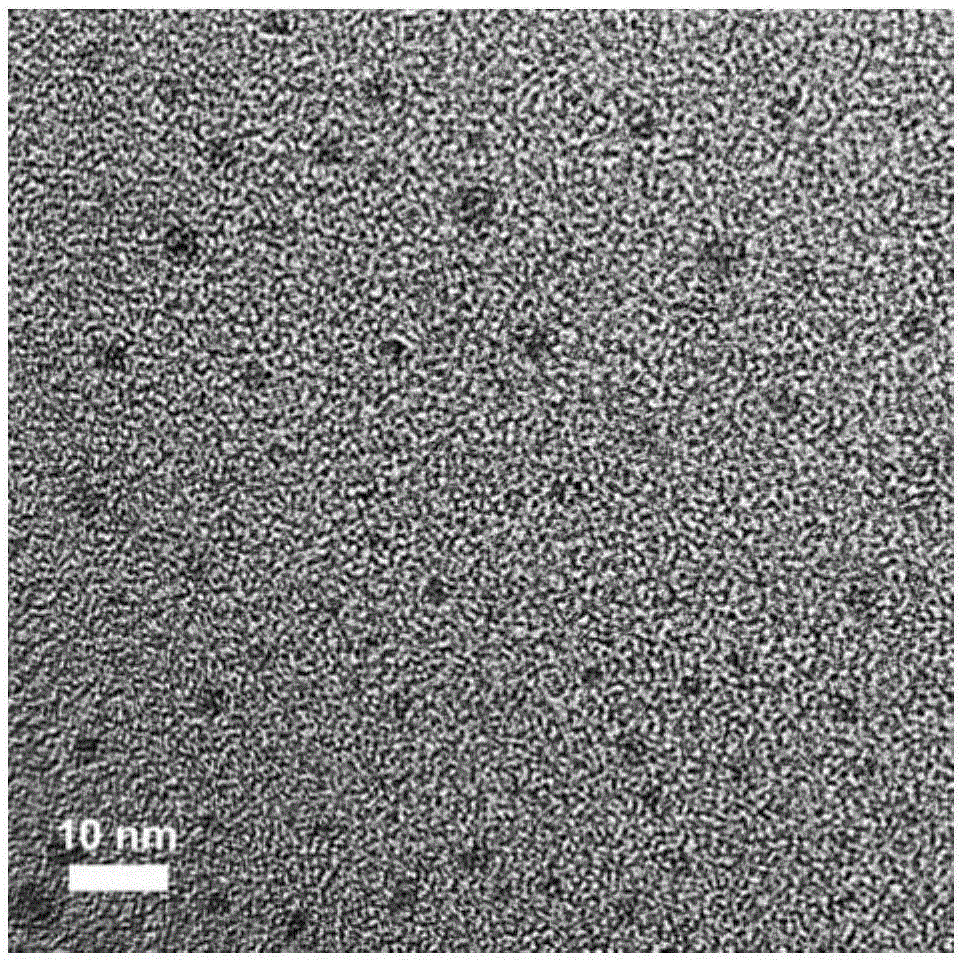
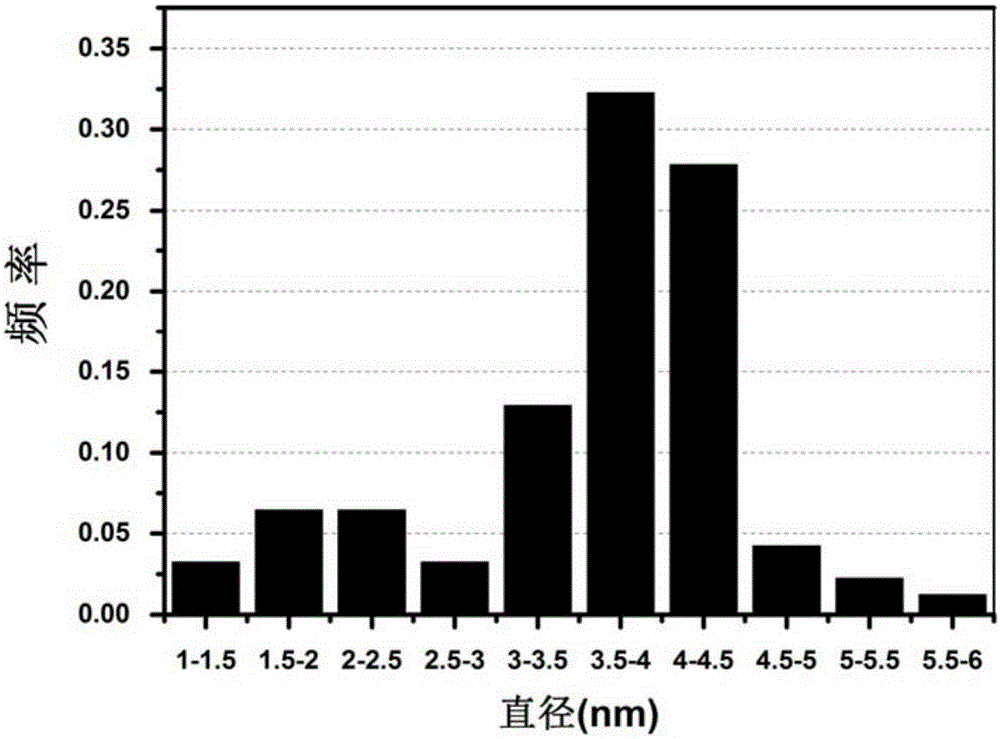
[0037] (2) Put the above-mentioned sealed hydrothermal reaction kettle into an environment of 150° C. to react for 18 hours to obtain the carbon quantum dots to be synthesized, such as figure 1 , 2 shown, from figure 1 , figure 2 It can be seen that the size of the prepared carbon quantum dots is below 6nm, from figure 2 It can be seen that 60% of the carbon quantum dot size prepared by the present invention is 3-5nm;
[0038] (3) Add the above-mentioned carbon quantum dot solution of 1ml respectively in 14 quartz fluorescence cuvettes;
[0039] (4) Use a pipette gun to adjust the concentration to 0; 2×10 -8 ;4×10 -8 ;6×10 -8 ;2×10 -7 ;4×10 -7 ;6×10 -7 ;8×10 -7 ;2×10 -6 ;4×10 -6 ;6×10 -6 ;2×10 -5 ;4×10 -5 ;6×10 -5 The FeCl3 solution joins in the above-mentioned cuvette that carbon quantum dot is housed, and it is put into the flu...
example 3
[0048] (1) prepare glucose and absolute ethanol mass ratio 25:1 dispersion liquid, place it in the hydrothermal reaction kettle and seal;
[0049] (2) Put the above-mentioned sealed hydrothermal reaction kettle into an environment of 180° C. to react for 18 hours to obtain the carbon quantum dots to be synthesized;
[0050] (3) Add the above-mentioned carbon quantum dot solution of 1ml respectively in 14 quartz fluorescence cuvettes;
[0051] (4) Use a pipette gun to adjust the concentration to 0; 2×10 -8 ;4×10 -8 ;6×10 -8 ;8×10 -8 ;2×10 -7 ;4×10 -7 ;6×10 -7 ;8×10 -7 ;2×10 -6 ;4×10 -6 ;6×10 -6 ;2×10 -5 ;4×10 -5 The FeCl3 solution joins in the above-mentioned cuvette that carbon quantum dot is housed, and it is put into the fluorescence spectrophotometer;
[0052] (5) After testing, the optimal excitation wavelength of the fluorescent carbon quantum dot solution in the present invention is 360nm, so the excitation wavelength of the fluorescence spectrophotometer is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com