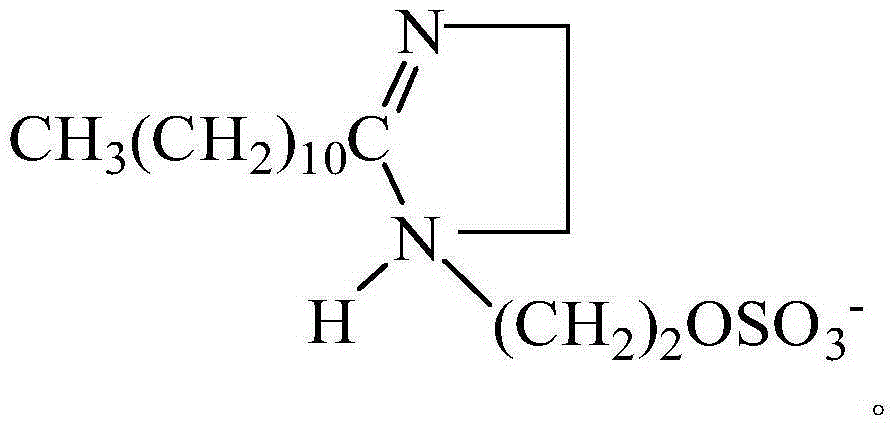
Imidazoline quaternary ammonium salt compound and preparation method therefor
A technology of imidazoline quaternary ammonium salts and compounds, which is applied in the field of imidazoline quaternary ammonium salt compounds and their preparation, can solve the problems of environmental friendliness and cost, complex preparation process of naphthenic acid, and great harm to production personnel, and achieve superiority Corrosion inhibition performance, low toxicity, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
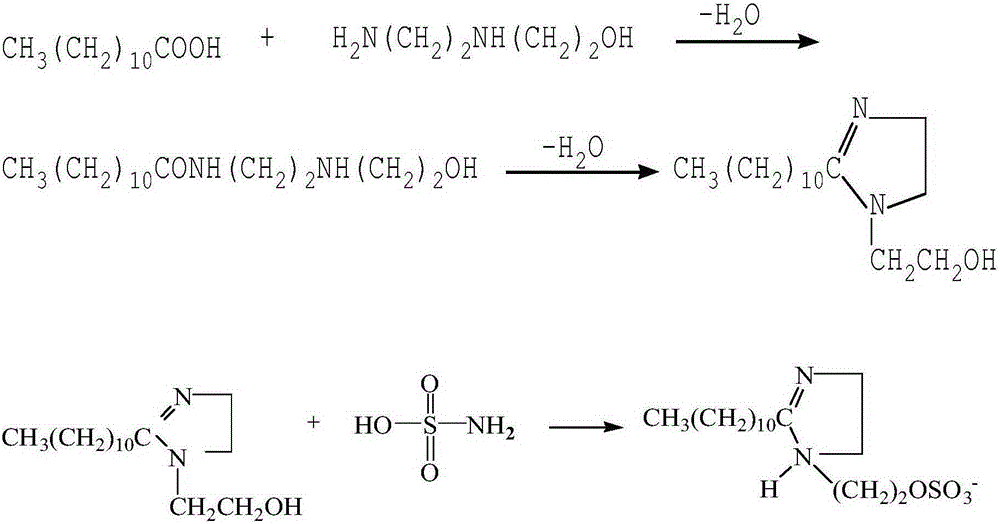
[0026] A kind of preparation method of imidazoline quaternary ammonium salt compound, comprises the following steps:
[0027] 1) Add lauric acid, hydroxyethylethylenediamine, water-carrying agent, catalyst and magnesium chips to the equipment equipped with reflux condenser, stirrer, thermometer, water separator and N 2 In the reactor of the protection device, the dehydration reaction is carried out at 150°C-160°C for 4-5 hours. During the reaction process, water can be seen to be carried out by the solvent. Dehydration reaction for 4-6 hours to obtain an imidazoline intermediate; recrystallize the imidazoline intermediate with acetone to obtain a purified imidazoline intermediate.
[0028] Among them, the molar ratio of lauric acid to hydroxyethylethylenediamine is 1: (1.1-1.3), the amount of water-carrying agent added is 20%-30% of the total mass of lauric acid and hydroxyethylethylenediamine, and the water-carrying agent The agent is xylene or toluene; the addition amount o...
Embodiment 1
[0034] The preparation of imidazoline quaternary ammonium salt compound comprises the following steps:
[0035] 1) Add lauric acid, hydroxyethylethylenediamine, xylene, Al 2 o 3 and magnesium scraps are added with a reflux condenser, agitator, thermometer, water separator and N 2 In the four-necked flask of the protection device, dehydration reaction was carried out at 150°C for 4 hours. During the reaction process, water was gradually taken out by the solvent, and the water output was basically stable after 4 hours of reaction. Then the temperature was raised to 210°C, and the dehydration reaction was carried out for 4 hours to obtain the imidazoline intermediate. ; After the imidazoline intermediate is distilled under reduced pressure and recrystallized from acetone, the purified imidazoline intermediate is obtained.
[0036] Wherein, the molar ratio of lauric acid and hydroxyethylethylenediamine is 1:1.1, and the amount of xylene added is 20% of the total mass of lauric a...
Embodiment 2
[0040] The preparation of imidazoline quaternary ammonium salt compound comprises the following steps:
[0041] 1) Add lauric acid, hydroxyethylethylenediamine, xylene, Al 2 o 3 and magnesium scraps are added with a reflux condenser, agitator, thermometer, water separator and N 2 In the four-neck flask of the protective device, dehydration reaction at 150°C for 4 hours, then heated up to 220°C, dehydration reaction for 5 hours, to obtain the imidazoline intermediate; after the imidazoline intermediate was distilled under reduced pressure and recrystallized from acetone, the purified imidazole was obtained Phenyl intermediates.
[0042] Wherein, the molar ratio of lauric acid and hydroxyethylethylenediamine is 1:1.2, and the amount of xylene added is 25% of the total mass of lauric acid and hydroxyethylethylenediamine; Al 2 o3 , The addition of magnesium chips is 0.2% of the quality of lauric acid.
[0043] 2) Add ethanol to the purified imidazoline intermediate, then heat ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com