Side-chain phthalonitrile modified benzoxazine resin, preparation method and application
A technology of phthalonitrile and benzoxazine, which is applied in the field of side-chain phthalonitrile modified benzoxazine resin and its preparation, can solve the problems of high brittleness of cured products, high curing reaction temperature and thermal stability Insufficient and other problems, to achieve the effect of improving thermodynamic performance, simple preparation method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
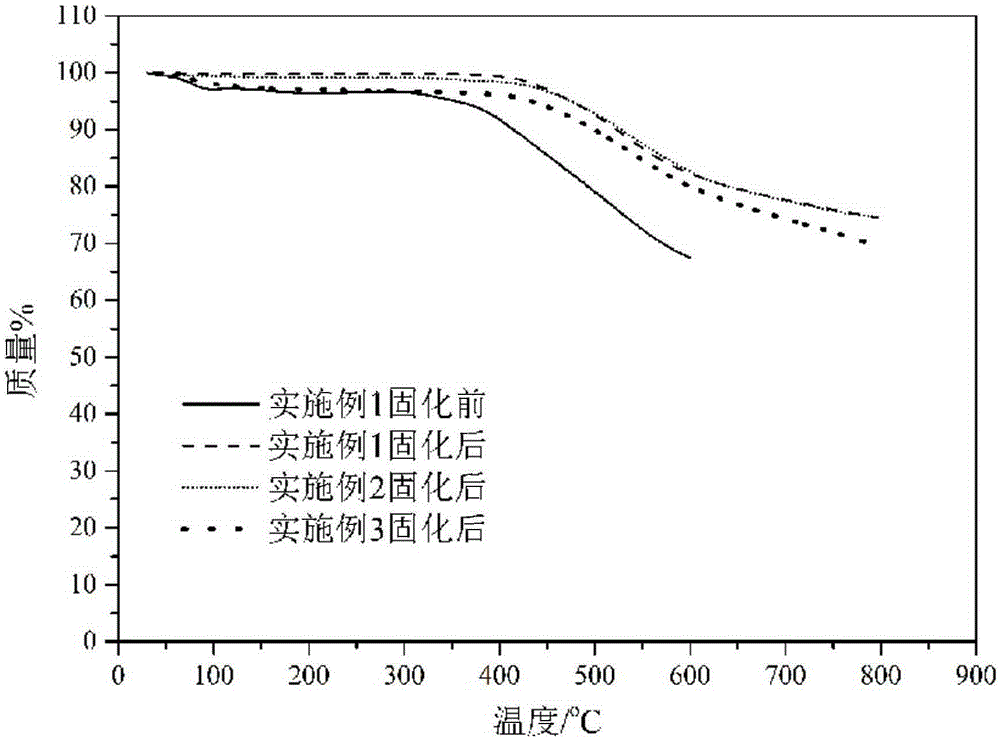
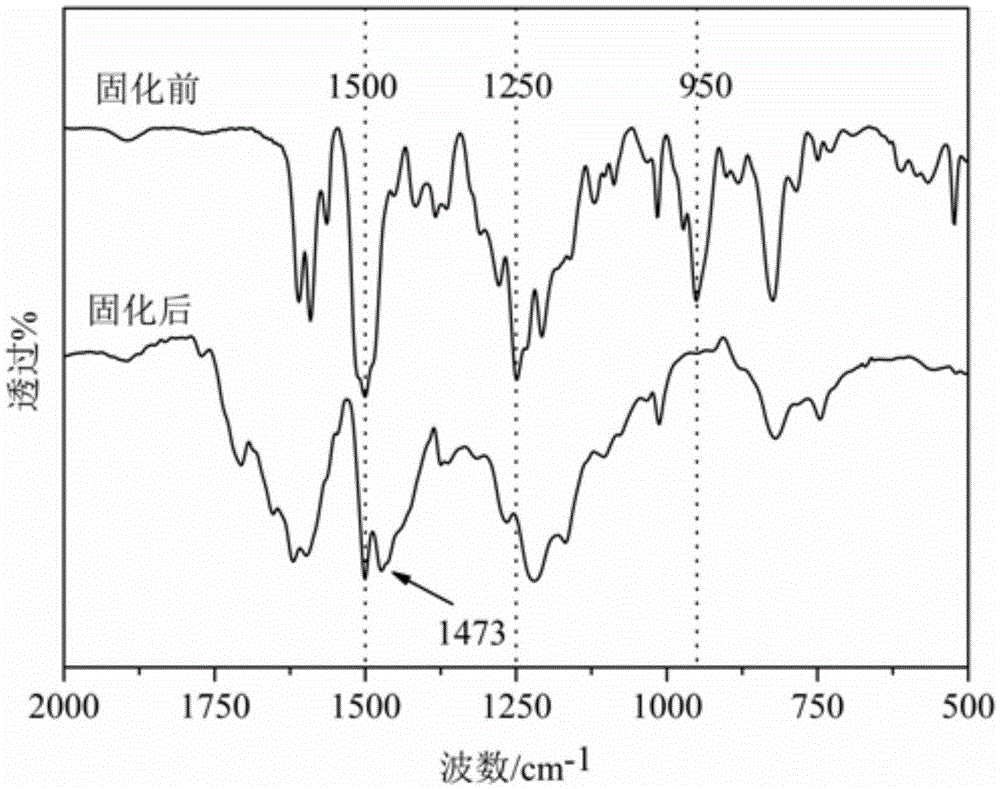
Embodiment 1
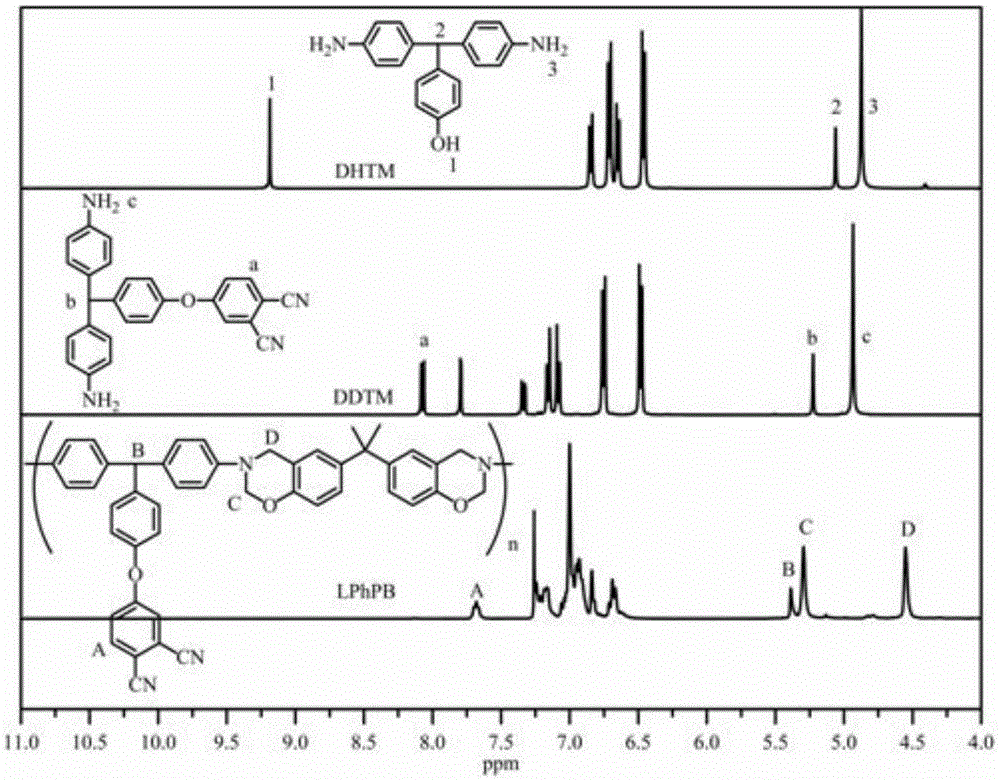
[0054] (1) Add 279g (3mol) of aniline, 122g (1mol) of p-hydroxybenzaldehyde and 13g of aniline hydrochloride in a 500mL flask equipped with a magnetic stirrer, then logical N 2 And gradually raise the temperature to 120° C. for 12 hours. After the reaction is completed, cool down to room temperature, add 200 mL of absolute ethanol, heat up and reflux for 2 hours again, filter after cooling down, wash the filtered solid with a small amount of absolute ethanol and water, remove aniline and aniline hydrochloride, and finally recrystallize 3 times with absolute ethanol 142 g of purple-red needle crystals were obtained, with a yield of 49%.
[0055] (2) In a 500mL flask equipped with a magnetic stirrer, add 14.66g (50.5mmol) of the compound of formula 2, 13.96g (101mmol) of anhydrous potassium carbonate, 8.66g (50mmol) of 4-nitrophthalonitrile and 250mL After the addition of acetonitrile, the temperature was gradually raised to 80°C for 12 hours. After the reaction was completed,...
Embodiment 2
[0060] (1) Add 279g (3mol) of aniline, 122g (1mol) of p-hydroxybenzaldehyde and 13g of aniline hydrochloride in a 500mL flask equipped with a magnetic stirrer, then logical N 2 And gradually raise the temperature to 150° C. for 6 hours. After the reaction was completed, the temperature was lowered to room temperature, 200 mL of absolute ethanol was added, and the temperature was raised to reflux for another 2 hours. After cooling down to room temperature, filter, and wash the filtered solid with a small amount of absolute ethanol and water to remove aniline and aniline hydrochloride, and finally recrystallize 3 times with absolute ethanol to obtain 116 g of purple-red needle-like crystals, with a yield of 40%.
[0061] (2) In a 500mL flask equipped with a magnetic stirrer, add 14.66g (50.5mmol) of the compound of formula 2, 13.96g (101mmol) of anhydrous potassium carbonate, 8.66g (50mmol) of 4-nitrophthalonitrile and 250mL of DMF , After the addition, react at room temperatur...
Embodiment 3
[0064] (1) Add 279g (3mol) of aniline, 122g (1mol) of p-hydroxybenzaldehyde and 13g of aniline hydrochloride in a 500mL flask equipped with a magnetic stirrer, then logical N 2 And gradually raise the temperature to 135° C. for 8 hours. After the reaction was completed and cooled down to room temperature, 200 mL of absolute ethanol was added, and the temperature was raised to reflux again for 2 hours. After cooling down to room temperature, filter and wash the filtered solid with a small amount of absolute ethanol and water to remove aniline and aniline hydrochloride, and finally recrystallize 3 times with absolute ethanol to obtain 122 g of purple-red needle-like crystals, with a yield of 42%.
[0065] (2) In a 500mL flask equipped with a magnetic stirrer, add 14.66g (50.5mmol) of the compound of formula 2, 13.96g (101mmol) of anhydrous potassium carbonate, 8.66g (50mmol) of 4-nitrophthalonitrile and 250mL of DMF , After the addition, react at 60°C for 24 hours. After the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com