Bromine-substituted aromatic compound modified polymer material, and preparation method and application thereof
A technology for aromatic compounds and polymer materials, applied in the field of polymer chemistry, can solve the problems of complex synthesis routes, high material costs, and restrictions on wide application, and achieve the effects of simple synthesis process, low synthesis cost and high reaction efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
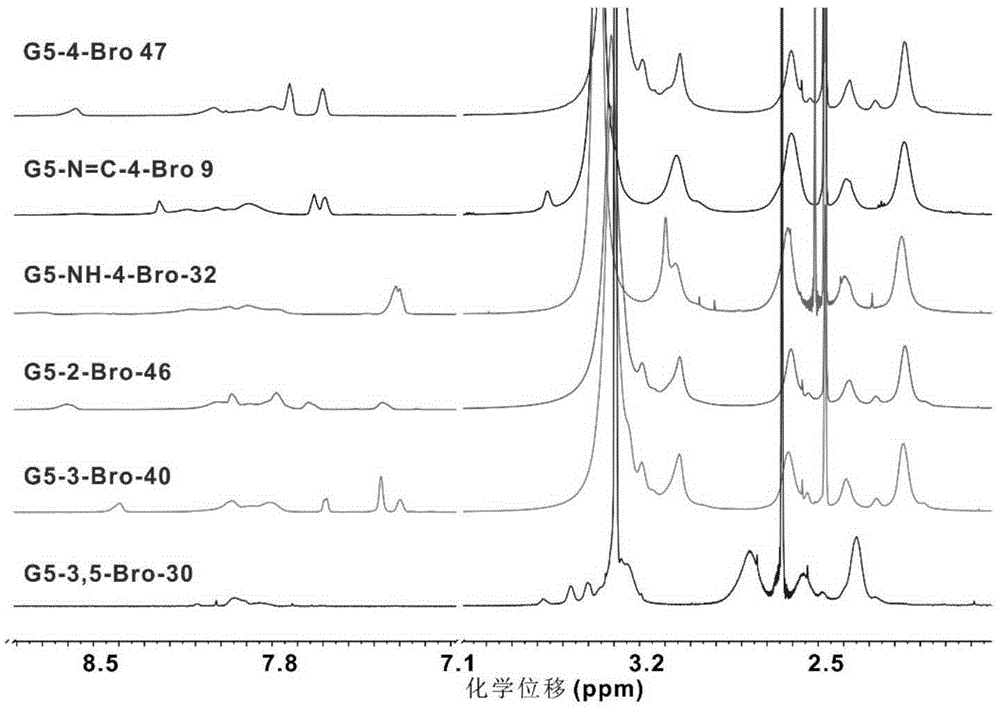
[0044] The present invention is based on the cationic polymer material of the fifth generation polyamido-amine dendrimers and bromine-substituted aromatic ring derivatives (G5-4-Bro-a, G5-N=C-4-Bro-a, G5 -NH-4-Bro-a, G5-2-Bro-a, G5--3-Bro-a, G5-3,5-Bro-a) preparation and characterization
[0045] (1) cationic macromolecule material G5-4-Bro-a based on the fifth generation polyamide-amine dendrimers and bromine-substituted aromatic ring derivatives as shown in reaction formula (I), its structural formula is as follows Formula (1A) shows:
[0046] The following reaction formula (I) cationic polymer material G5-4-Bro-a, G5-N=C-4-Bro-a, G5-NH-4-Bro-a, G5-2-Bro-a, G5- -3-Bro-a, G5-3,5-Bro-a synthetic route and structural formula:
[0047]
[0048] Among them, G5 is the fifth generation polyamide-amine dendrimer, the central core is ethylenediamine, and the terminal group is a primary amine group. In formula (2), n is 6, m is 2, and its structure is as follows: (3A);
[0049]...
Embodiment 2
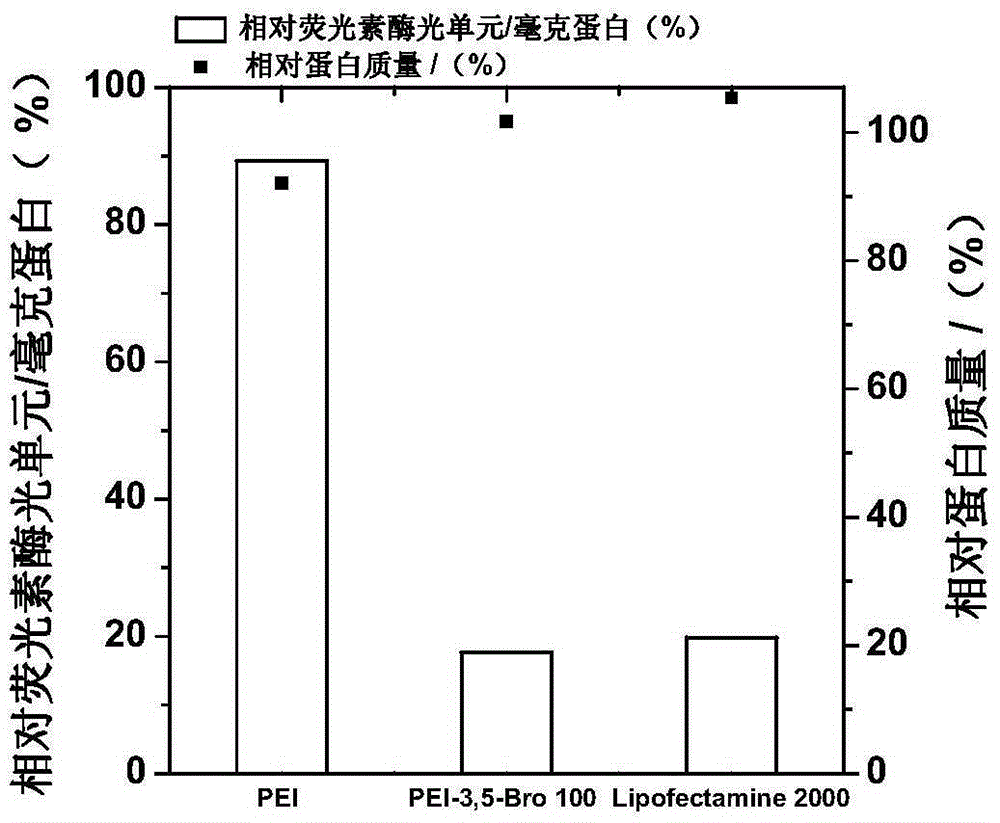
[0116] The present invention is based on the gene transfection material of polyethylenimine cationic polymer and bromine-containing aromatic ring derivative, that is, the polyethyleneimine cationic polymer polymer material (PEI-2-Bro-a) modified by bromine-containing aromatic ring derivative , PEI-3-Bro-a, PEI-3,5-Bro-a) preparation and characterization.
[0117] (1) As shown in the reaction formula (II), it is a gene transfection material based on polyethyleneimine cationic polymer and bromine-containing aromatic ring derivatives, that is, the polyethyleneimine cationic polymer modified by bromine-containing aromatic ring derivatives is high The reaction equation of the molecular material PEI-2-Bro-a,
[0118]
[0119] That is, in the formula (1), when R is a branched polyethyleneimine (bPEI25KD) of 25KD, the structural formula of the polymer material is as shown in the following formula (2A):
[0120]
[0121] in,
[0122] a represents the quantity that 2-bromobenzoi...
Embodiment 3
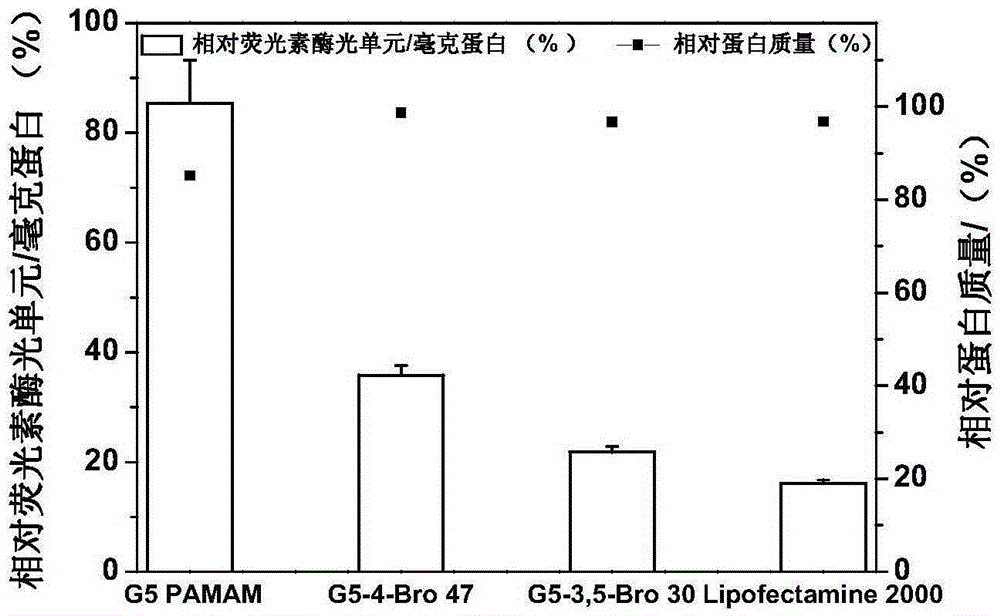
[0144] Example 3: Gene knockout experiment of gene transfection materials G5-4-Bro-47 and G5-3,5-Bro-30 in HeLa-luc cells
[0145] Compound the gene knockout reagents G5-4-Bro-47 and G5-3,5-Bro-30 prepared in Example 1 with siRNA (siLuc) that can specifically knock out the luciferase gene at room temperature and knockout the gene in HeLa-luc cells. The gene knockout efficiency was evaluated by detecting the expression level of luciferase, and the total protein amount of the cells was used as an indicator for evaluating the toxicity of the material. The specific method is: culture HeLa-luc cells in a 24-well plate for 24 hours, mix 0.5ugsiLuc with G5-4-Bro-47 or G5-3,5-Bro-30 in different mass ratios (w / w =1:1-20:1), add serum-free medium to make the total volume 100ul, half an hour later, add 150ul serum-free medium, add the medium containing the complex to the cells, and incubate After 6 hours, add 500 microliters of medium containing 10% serum, continue to cultivate for 18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com