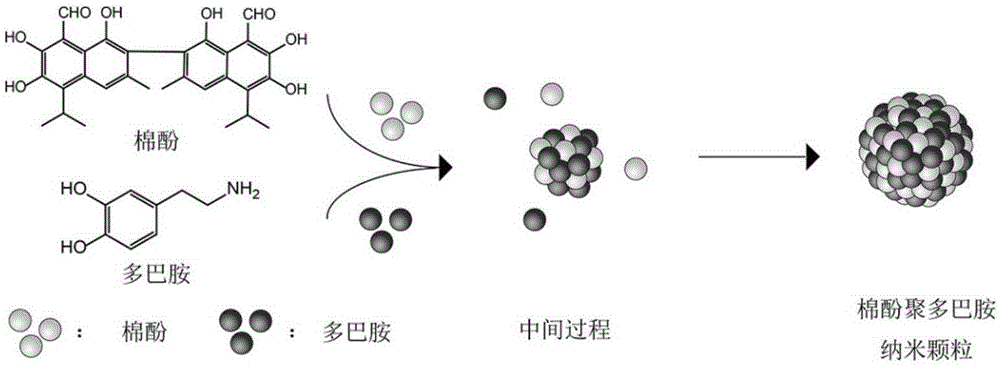
Method of preparing gossypol and its derivative polydopamine nano-carrier by polymerization process
A technology of polydopamine nano and derivatives, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc., to achieve the effect of improving water solubility, high encapsulation rate, and broadening the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] refer to figure 1 , this embodiment includes the following steps:
[0025] Step 1: Dissolve 0.3mg gossypol acetate in 0.5mL methanol, disperse under 100kHz ultrasound, and the dispersion time is 3 minutes to make a mixed solution;
[0026]Step 2: Add the mixed solution dropwise to 10 mL of tris-hydrochloric acid buffer solution with a pH value of 9 at a rate of 4 drops per minute while stirring, and the stirring speed is 200 rpm. , keep the speed and continue stirring for 5 minutes;
[0027] Step 3: Add 3.0 mg of dopamine to the above system, and keep stirring at room temperature for 4 hours;
[0028] Step 4: Put the obtained product into a dialysis bag with a molecular weight cut-off of 1000 Daltons, and dialyze in tris-hydrochloric acid buffer for 24 hours to remove uncoated drugs.
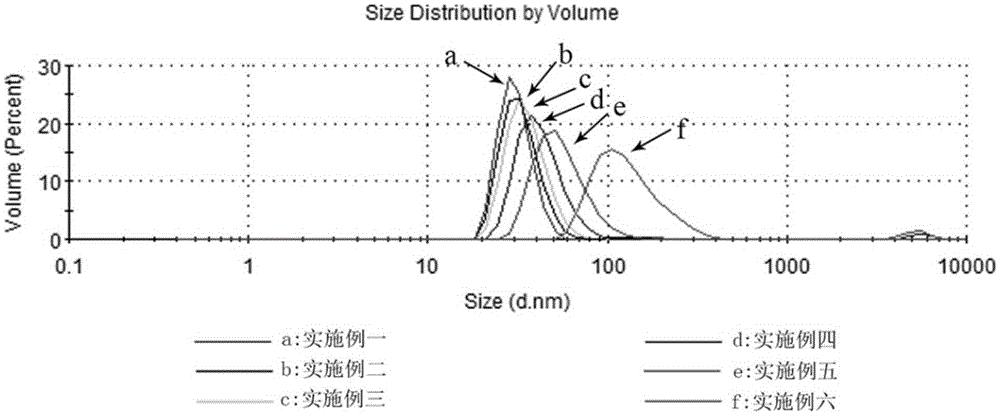
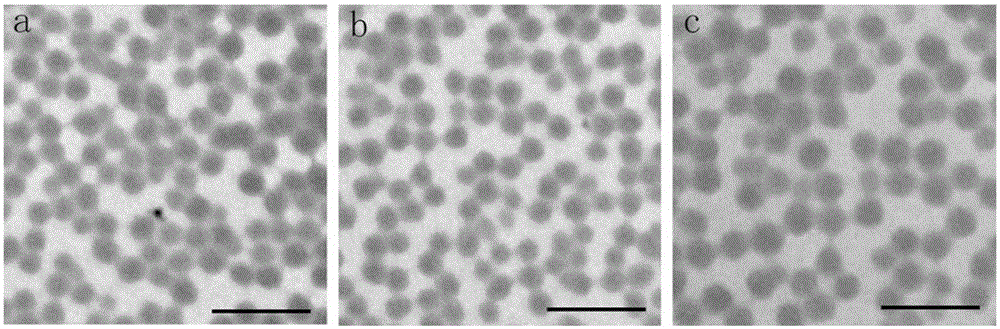
[0029] The particle size of the nanocarrier obtained in this embodiment is measured by a Malvern particle size analyzer, referring to figure 2 Middle a is the measurement result of t...
Embodiment 2
[0032] This embodiment includes the following steps:
[0033] Step 1: Dissolve 0.6mg gossypol acetate in 0.5mL ethanol, disperse under 100kHz ultrasound, and disperse for 30 minutes to make a mixed solution;
[0034] Step 2: Add the mixed solution dropwise to 20 mL of a 1 mg / mL sodium hydroxide dilute solution with a pH value of 10 at a rate of 8 drops per minute while stirring, and the stirring speed is 300 rpm. After the dropwise addition is completed, Keep stirring continuously for 10 minutes;
[0035] Step 3: Add 6.0 mg of dopamine to the above system, and keep stirring at room temperature for 8 hours;
[0036] Step 4: Put the obtained product into a dialysis bag with a molecular weight cut-off of 1000 Daltons, and dialyze in the same weak alkaline buffer as in Step 2 for 24 hours to remove uncoated drugs.
[0037] The particle size of the nanocarriers obtained in this example was measured by a Malvern particle size analyzer. refer to figure 2 Middle b is the measurem...
Embodiment 3
[0040] This embodiment includes the following steps:
[0041] Step 1: Dissolve 1.0mg of apogossypol in 1.0mL of ethanol, and disperse under 100kHz ultrasound for 5 minutes to make a mixed solution;
[0042] Step 2: Add the mixed solution dropwise to 25 mL of disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution with a pH value of 8.5 while stirring at a rate of 20 drops per minute, the stirring speed is 400 rpm, and the addition is completed After that, keep the speed and continue to stir for 10 minutes;
[0043] Step 3: Add 10 mg of dopamine to the above system, and keep stirring at room temperature for 15 hours;
[0044] Step 4: Put the obtained product into a dialysis bag with a molecular weight cut-off of 1000 Daltons, and dialyze in the same weak alkaline buffer as in Step 2 for 48 hours to remove uncoated drugs.
[0045] The particle size of the nanocarrier obtained in this embodiment is measured by a Malvern particle size analyzer, referring to fig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com