Method for extracting methylnaphthalene in C10 aromatics
A technology for carbon ten aromatic hydrocarbons and methyl naphthalene is applied in the field of extracting methyl naphthalene from carbon ten aromatic hydrocarbons, which can solve the problems of low methyl naphthalene purity and yield, large equipment investment and high production cost, and achieves short cycle time and energy consumption. Low, high relative yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
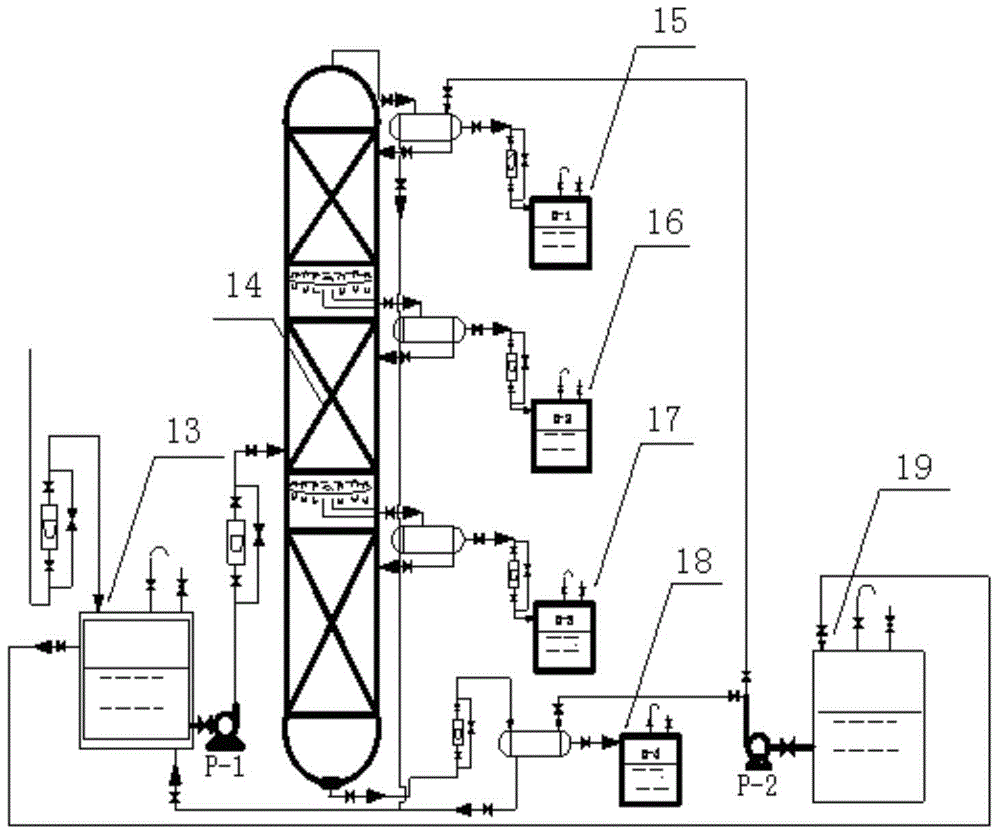
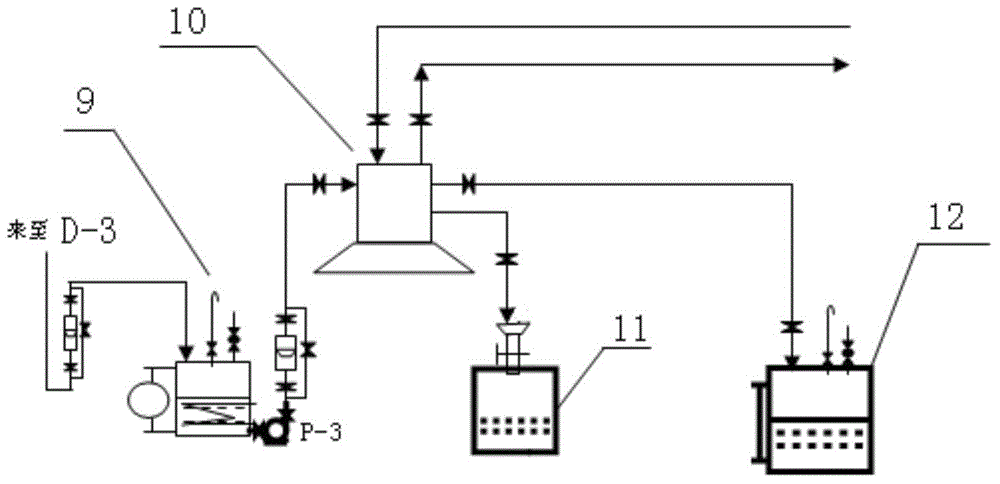
[0031] The process of extracting methyl naphthalene from carbon deca aromatic hydrocarbons by the integrated method of continuous lateral distillation and drum crystallization, refer to figure 1 , figure 2 :
[0032] (1) The continuous intermediate feeding method is adopted, and the raw materials are pumped from the carbon deca aromatic hydrocarbon storage tank into the sideline rectification tower kettle, and heating starts when the liquid level reaches 1 / 3;
[0033] (2) When the temperature at the top and bottom of the tower becomes stable and the device reaches full reflux, the temperature at the top D-1 is 203.5-206.1℃, the temperature at the side line D-2 is 221.5-223.6℃, and the temperature at the side line D-3 is 244.7~246.5℃, the temperature of tower reactor D-4 is 252.5~256.6℃;
[0034] (3) After separation, the top component is light carbon ten component, the side line D-2 component is mesitylene and naphthalene enriched liquid; the side line D-3 component is methyl naph...
Embodiment 2
[0043] The process of extracting methyl naphthalene from carbon deca aromatic hydrocarbons by the integrated method of continuous lateral distillation and drum crystallization, refer to figure 1 , figure 2 . The inlet and outlet flow rate, feeding position, reflux ratio and crystallizer temperature of the side-line rectification tower are shown in Table 3. Other conditions are the same as in Example 1, and the experimental results are shown in Table 4.
[0044] Table 3 Continuous sideline distillation and drum crystallization process conditions
[0045]
[0046] Table 4 Results of extraction of methyl naphthalene from carbon deca aromatics by continuous lateral distillation and drum crystallization
[0047]
Embodiment 3
[0049] The process of extracting methyl naphthalene from carbon deca aromatic hydrocarbons by the integrated method of continuous lateral distillation and drum crystallization, refer to figure 1 , figure 2 . The feed and discharge flow rate, feed position, reflux ratio and crystallizer temperature of the sideline rectification tower are shown in Table 5. Other conditions are the same as in Example 1, and the experimental results are shown in Table 6.
[0050] Table 5 Continuous sideline distillation and drum crystallization process conditions
[0051]
[0052] Table 6 Results of extraction of methyl naphthalene from carbon deca aromatics by continuous lateral distillation and drum crystallization
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com