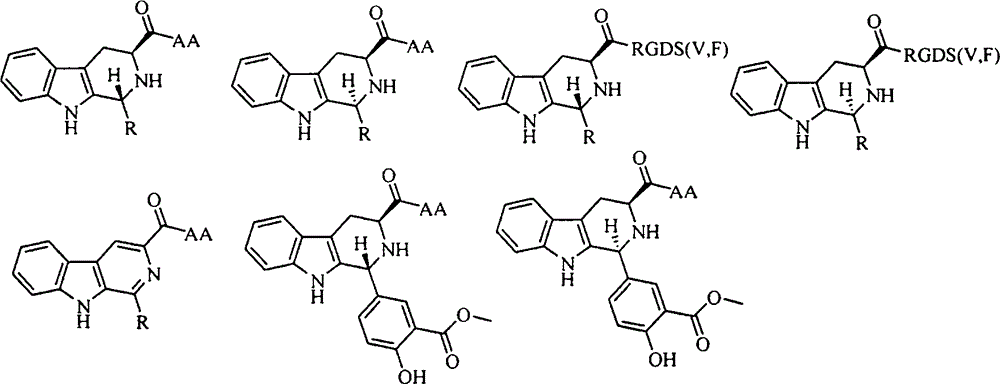
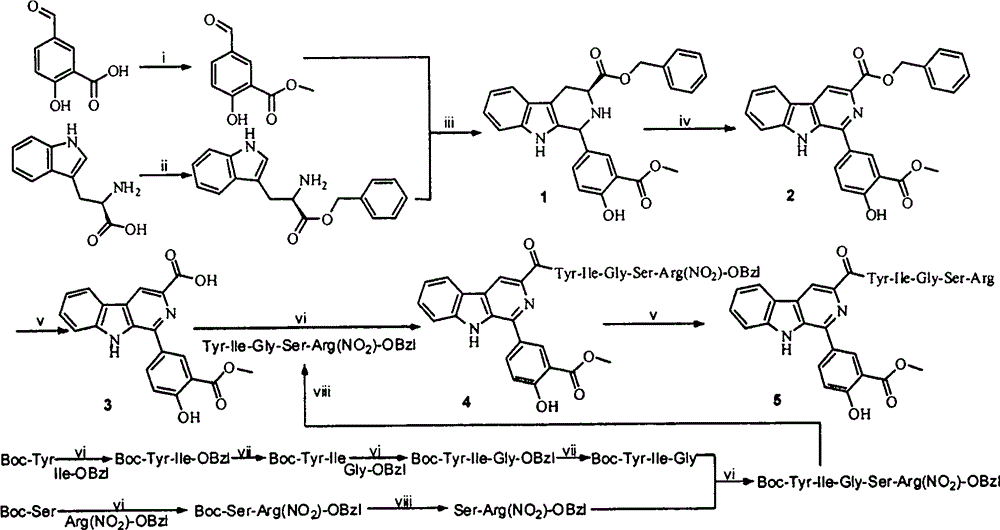
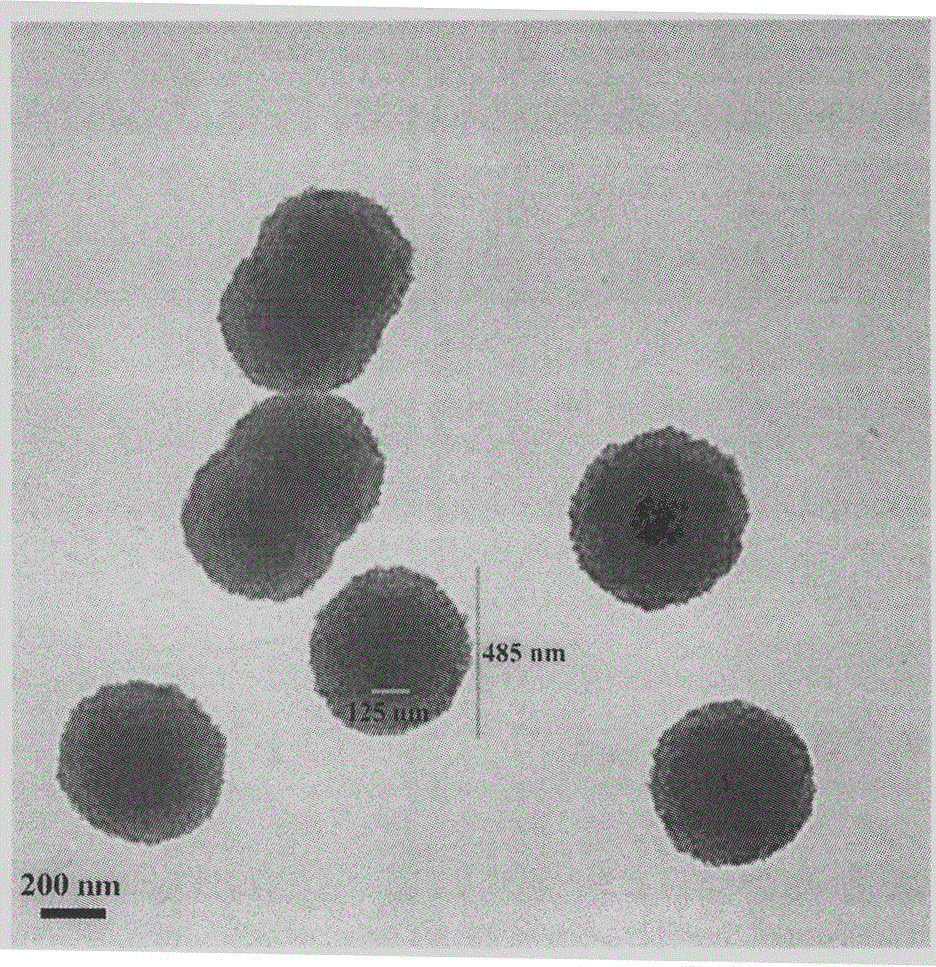
YIGSR-modified beta-carboline, preparation, nano-structure, activity and application thereof
A carboline and reactive technology, applied in the field of anti-inflammatory and anti-thrombotic effects, can solve the problems of anti-tumor, anti-inflammatory, anti-thrombotic and anti-tumor adhesion, invasion and migration compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 prepares methyl 5-formylsalicylate
[0030] Weigh 1.660g (10.0mmol) of 5-formylsalicylic acid in a microwave reaction tank, add 25mL of methanol and 1mL of concentrated H 2 SO 4 , reacted in a microwave reactor at 90°C for 2 hours, monitored by TLC until the raw material spots disappeared, stopped the reaction and cooled down to room temperature, transferred the reaction solution to a 100mL eggplant-shaped bottle, adjusted the pH value to 7-8 with concentrated ammonia water, and reacted After the solution was concentrated to dryness under reduced pressure, a large amount of ethyl acetate was added to dissolve it, and the ethyl acetate layer was successively washed with saturated NaHCO 3 , saturated NaCl three times each, and then washed with anhydrous NaCl 2 SO 4 It was dried for 2 hours, filtered, and concentrated under reduced pressure. After standing at room temperature overnight, crystals precipitated to obtain 1.635 g (90.8%) of the title compound a...
Embodiment 2
[0031] Embodiment 2 prepares L-tryptophan benzyl ester
[0032]Weigh 15.0g (44.4mmol) of polyphosphoric acid in a 500mL eggplant-shaped bottle, add 80mL of benzyl alcohol, and dissolve it in an oil bath at 50°C. After the temperature of the solution rises to 75°C, weigh 10g (49.0mmol) of L - Add tryptophan to it, react at 75°C for 48 hours, use TLC to monitor until the raw material spots disappear, stop the reaction and cool down, pour 400mL of anhydrous ether into the reaction bottle under stirring in an ice bath, at this time a white solid precipitates, stir After overnight, filter it, suspend the white solid with 200mL ethyl acetate and 10mL water, adjust the pH value of the solution to about 8 with triethylamine, the solution becomes clear, let it stand for liquid separation, and wash the separated ester layer with saturated NaHCO 3 , saturated NaCl three times each, and the ethyl acetate layer was washed with anhydrous NaCl 2 SO 4 Dry for 2 h, filter, and concentrate to...
Embodiment 3
[0033] Example 3 Preparation of 1-(4-hydroxyl-3-methoxycarbonylphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1)
[0034] Add 100mLCH to a 250mL eggplant-shaped bottle 2 Cl 2 And 10mLTFA, after stirring evenly, weigh 11.76g (40.0mmol) L-tryptophan benzyl ester and 7.92g (44.0mmol) methyl 5-formylsalicylate and add it, and the reaction solution becomes reddish after a few minutes. After 2 days, the reaction solution turned black. Slowly add concentrated ammonia water dropwise under ice-bath stirring to adjust the pH value of the reaction solution to 8. The reaction solution was allowed to stand for liquid separation, and the CH 2 Cl 2 Layers were sequentially washed with saturated NaHCO 3 and saturated NaCl three times each, CH 2 Cl 2 Anhydrous Na 2 SO 4 Dry for 2 h, filter, and concentrate to dryness under reduced pressure to give 14.59 g (80%) of the title compound as a yellow solid. ESI-MS(m / e): 457[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com