Recombined ULP1 kinase coding sequence, coding protein, plasmid containing recombined ULP1 kinase coding sequence and recombined ULP1 kinase preparation method
A coding sequence and plasmid technology, applied in the field of molecular biology, can solve the problems of complex preparation process and low purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation process of recombinant ULP1 kinase
[0028] 1. According to the codon usage preference of E. coli, for ULP1 (403-621) The coding genes corresponding to this part of the protein were optimized. The specific optimized sequence is shown in SEQ ID NO.1, and a 6xHis tag is introduced at the end of the sequence.
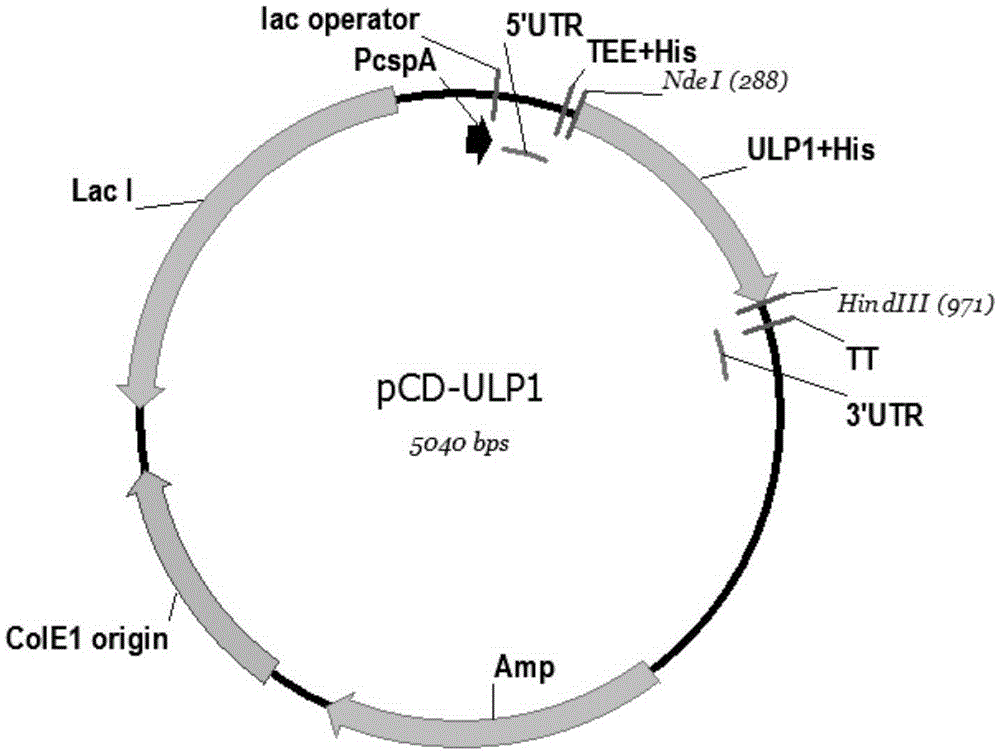
[0029] 2. Carry out the whole gene synthesis of the sequence corresponding to SEQIDNO.1, and connect the synthesized SEQIDNO.1 into the commercial expression vector pCold-II (the vector is self-sustained at the front end of NdeI) by enzyme digestion (NdeI and HindIII) connection. with 6xHis sequence), named pCD-ULP1, and the sequence is shown in SEQ ID NO.2. like figure 1 As shown, at this time both N and C ends of ULP1 kinase have 6xHis tags (6xHis-ULP1 (403-621) -6xHis), the recombinant ULP1 kinase with this structure has a very high affinity to the nickel column, so it can be eluted with 200mM imidazole, and finally the recombinant ULP1 ...
Embodiment 2
[0038] Example 2 Effects of Different Temperatures on the Heterologous Expression of Recombinant ULP1 Kinase
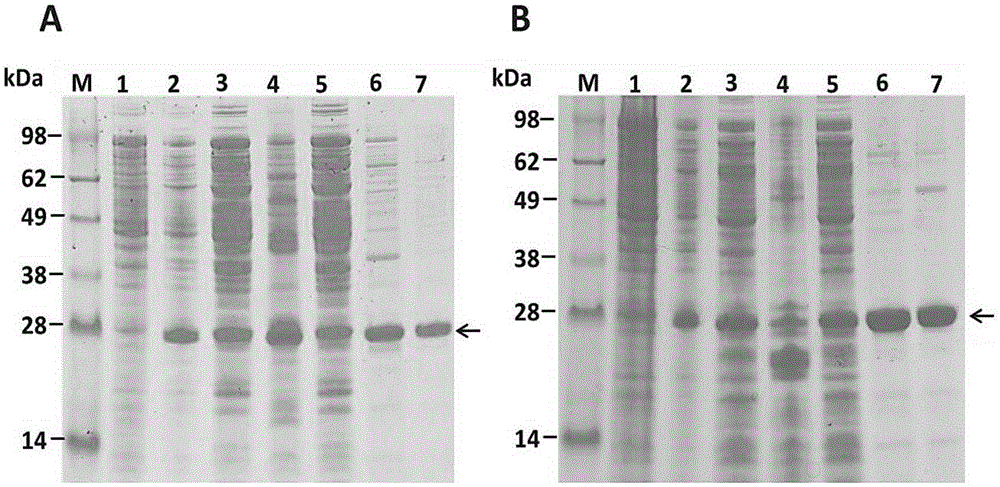
[0039] The inventors also explored the expression of recombinant ULP1 kinase in Escherichia coli at different temperatures. It was found that the recombinant ULP1 kinase has a good expression effect at a very wide range of temperatures (16-37 degrees) ( figure 2 ), which means that researchers can choose the appropriate temperature according to the needs of the experimental progress. figure 2 shows the expression of recombinant ULP1 kinase at 16 degrees and 37 degrees, and found that at these two temperatures, recombinant ULP1 kinase can be clearly expressed. Simultaneously, the inventor also explored other temperatures such as 30 degrees, 25 degrees, 20 degrees, etc., and the results were basically consistent with 37 degrees. The lower the temperature, the slower the growth of the bacteria, so the induction time will be extended as the temperature decreases, and ...
Embodiment 3
[0040] Example 3 Activity Detection of Recombinant ULP1 Kinase
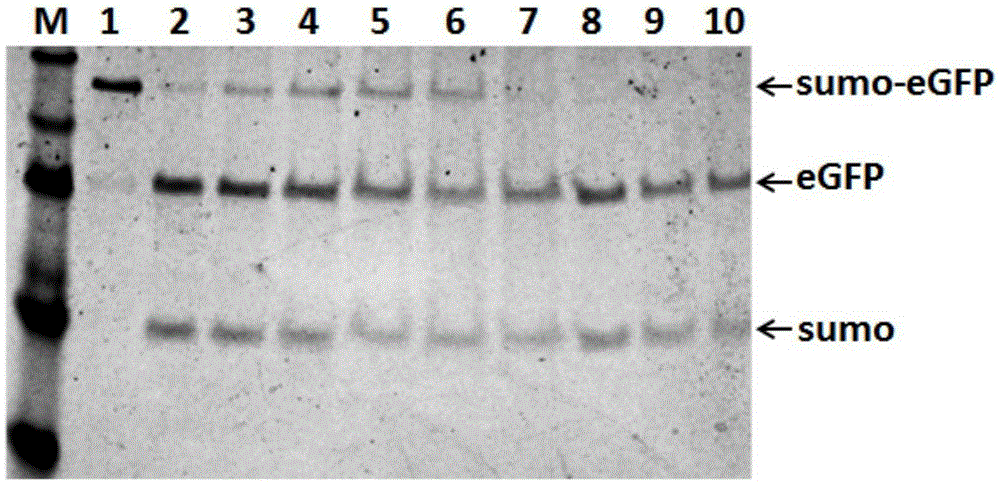
[0041] The inventors compared the cleavage efficiency of the recombinant ULP1 kinase under different conditions, such as image 3 As shown, it is found that the recombinant ULP1 kinase prepared by this method has very high activity in its cutting standard buffer, Tris-HCl, PBS buffer, and even pure water, and can completely cut the substrate protein. The standard reaction system is: total volume 50 μl, 5 μg substrate (sumo-eGFP), 1 μl prepared recombinant ULP1 kinase.
[0042] Standard cleavage buffer composition: 25mM Tris-HCl, 1% NP-40, 250mM NaCl, 50% (V / V) Glycerol, pH=8.0.
[0043] Tris-HCl buffer composition: 25mM Tris-HCl, pH=8.0.
[0044] PBS buffer composition: 38.7mMNa 2 HPO 4 ,11.3mMNaH 2 PO 4 , 150mM NaCl, pH=7.4.
[0045]
[0046]
[0047]
[0048]
[0049]
[0050]
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com