Establishment of mouse tuberculosis natural dormant infection model preparation and drug evaluation methods
A technology of latent infection and mice, which is applied in the field of mouse tuberculosis natural latent infection model preparation and drug evaluation method establishment, can solve the problems of inability to study the immune mechanism, long incubation period, long research cycle, etc., and achieve convenient latent and relapse mechanism , the effect of stabilizing the acute phase and shortening the incubation period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The construction of embodiment 1 mouse tuberculosis latent infection model
[0036] SPF-grade C57 mice (6-8 weeks old, female, 450 mice) were purchased from Victoria Lihua Company and adapted to ABSL-3 for 1 week. All mice were raised in accordance with the ethical requirements of animal welfare, and obtained the approval number ILAS-PC-2013-015 from the Animal Welfare Ethics Committee of the Institute of Medical Experimental Animals, Chinese Academy of Medical Sciences. After the mice entered the ABSL-3 laboratory, 6 mice per cage, free access to water and food, laboratory humidity (50±10%), light (12 / 12h light / dark cycle), temperature (23±2°C). At the end of the experiment, mice were fixed and euthanized.
[0037] Mycobacterium tuberculosis H37Rv grows on the L-J medium for 3 weeks to the logarithmic growth phase, add 2ml of 0.9% NaCl, scrape off the tuberculosis colonies with a sterile L glass rod, blow and mix them repeatedly with a 1ml pipette, and pass the bacter...
Embodiment 2
[0040] The detection of the lung of embodiment 2 mouse latent infection model of tuberculosis, spleen tissue bacteria load
[0041] 1, 3, 5, 8, 12, 16, 20 and 24 weeks after the infection of Example 1, 6 mice were dissected respectively, and the lung and spleen tissues were put in 4% H 2 SO 4 Remove possible miscellaneous bacteria, put the tissue in a 2ml glass grinder, add 1ml 0.9% NaCl for grinding. The homogenate was serially diluted 10 times, and 100 μL of the homogenate was inoculated on the L-J slope, and placed at 37°C, 5% CO 2 cultured in an incubator. After 21 days counting, 6 mice per time point were subjected to statistical analysis. (T-test)
[0042] The bacterial loads of lung and spleen tissues at 1, 3, 5, 8, 12, 16, 20 and 24 weeks after infection are shown in Table 1:
[0043] Table 1 Bacteria load in mouse lung and spleen tissue
[0044]
[0045] Note: *** shows significant difference in the number of sclerotia compared with the blank control group (P...
Embodiment 3
[0048] Lung and spleen histopathological detection of embodiment 3 mouse tuberculosis latent infection model
[0049] Example 1 Dissect 6 mice at 1, 3, 5, 8, 12, 16, 20, and 24 weeks after infection, and dissect 6 mice at each time point at 34, 38, 44, and 52 weeks after infection in the first experiment , while the second and third experiments, the remaining mice after 24 weeks were dissected at 52 weeks. The lung, spleen and liver tissues of the mice were dissected, checked for gross pathology, and soaked in formalin. According to the routine pathological embedding and repair method, HE staining was performed after sectioning, and histopathological lesions were diagnosed by senior animal pathology experts, and pathological scoring was performed. According to the size of the lesion and the degree of inflammation, it was divided into: +, 25%; ++ ,50%; +++,75% of the 3 grades.
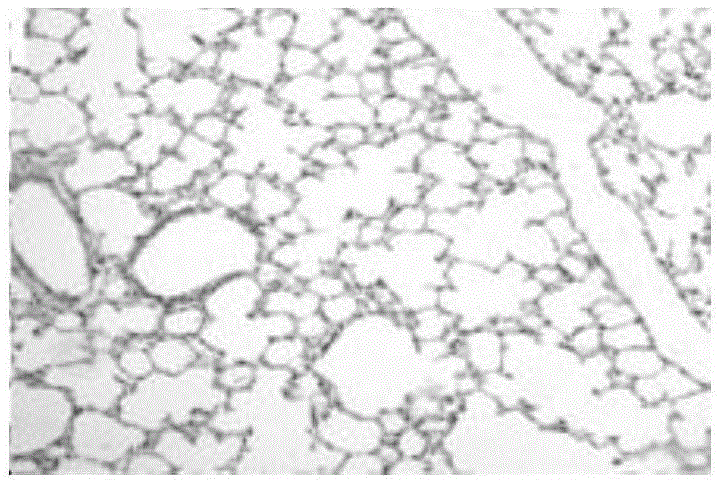
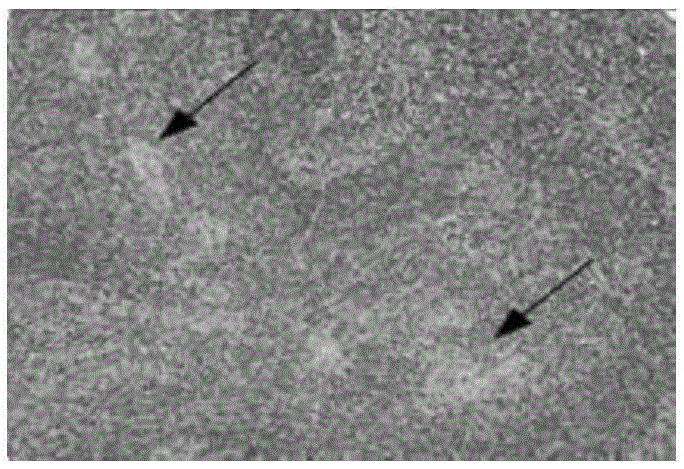
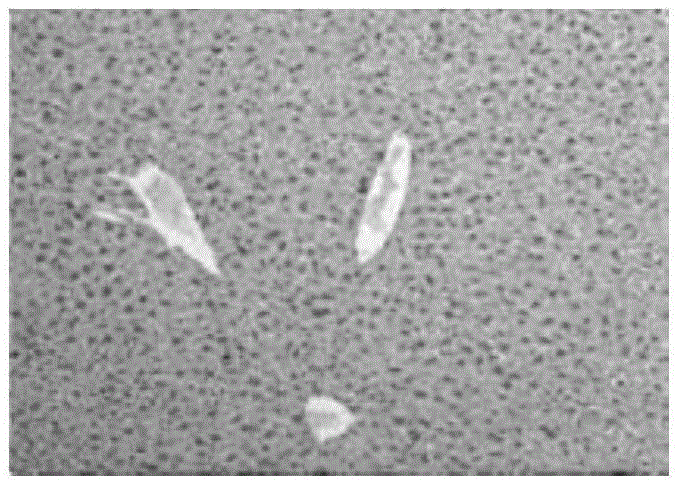
[0050] Throughout the experimental period, there were no visible lesions in the lung and spleen ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com