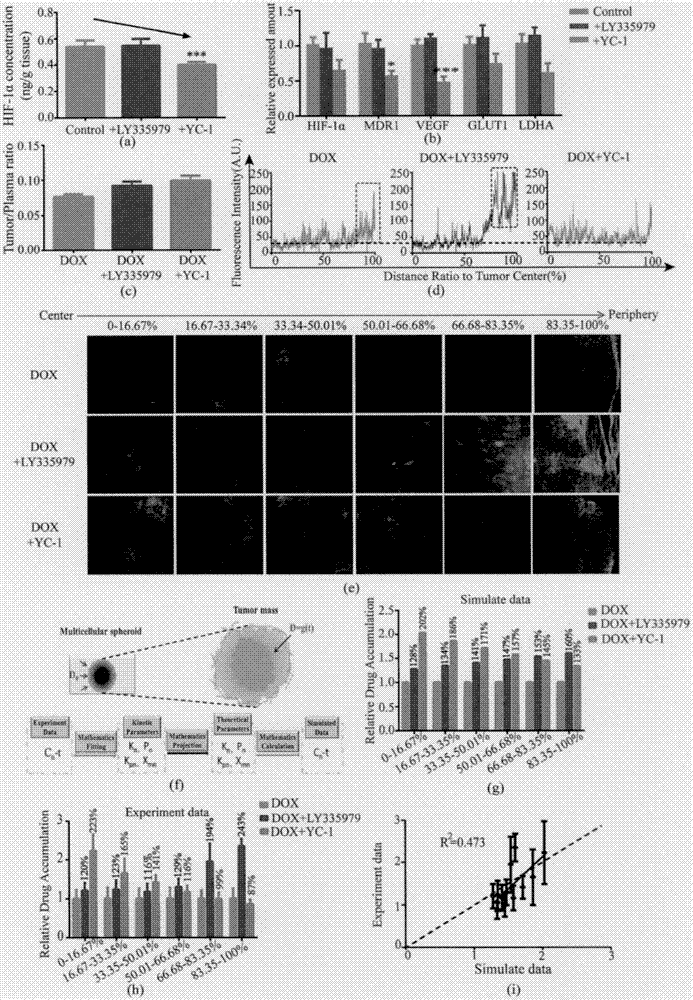
Patents
Literature
65 results about "Drugs evaluations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for establishing humanized rat drug evaluation animal model
InactiveCN104593418AVector-based foreign material introductionAnimal husbandryLarge fragmentEngineered genetic
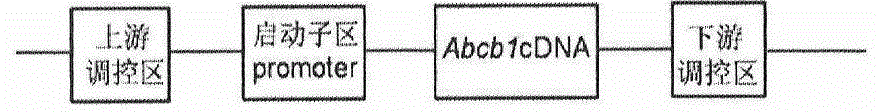
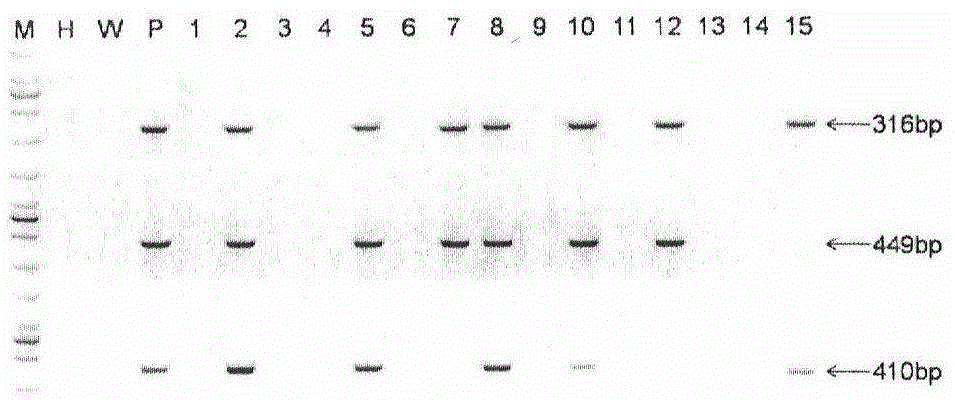
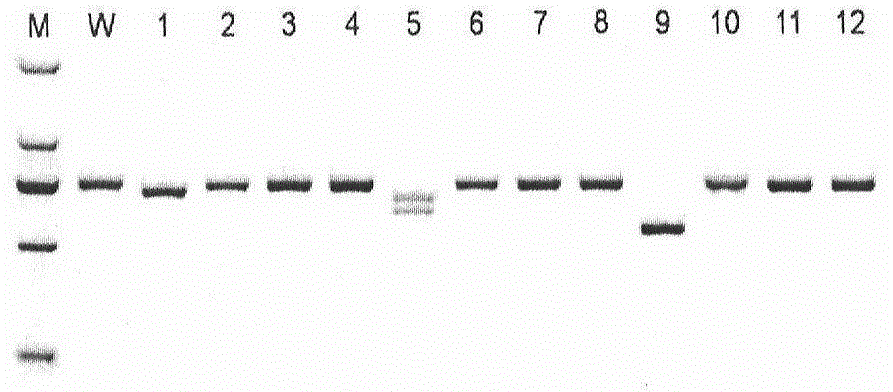
The invention provides a method for establishing a humanized rat drug evaluation animal model. According to the method, a multidrug resistance gene 1 (Abcb1)-knocked-out genetically engineered rat is obtained through a microinjection method by virtue of a CRISPR / Cas9 gene knockout technology and 153kb bacterial artificial chromosome (BAC) fragments containing a humanized Abcb1 promoter and cDNA is simultaneously inoculated into the rat genome through the microinjection method by virtue of a large fragment transgenic technology to obtain a transgenic rat capable of stably expressing human Abcb1 and the genetically engineered rat and the transgenic rat are hybridized to establish the humanized rat drug evaluation animal model. RT-PCR analysis shows that Abcb1 expression profiles of humanized Abcb1 rat are significantly different from those of the rat endogenous Abcb1. The method has the beneficial effects that the humanized rat capable of expressing human Abcb1 is obtained and the rat is used for expressing human Abcb1 genes and has closer expression profiles to those of human so that the model can be well used for the efficacy evaluation of newly developed drugs.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Modulation neural pathways
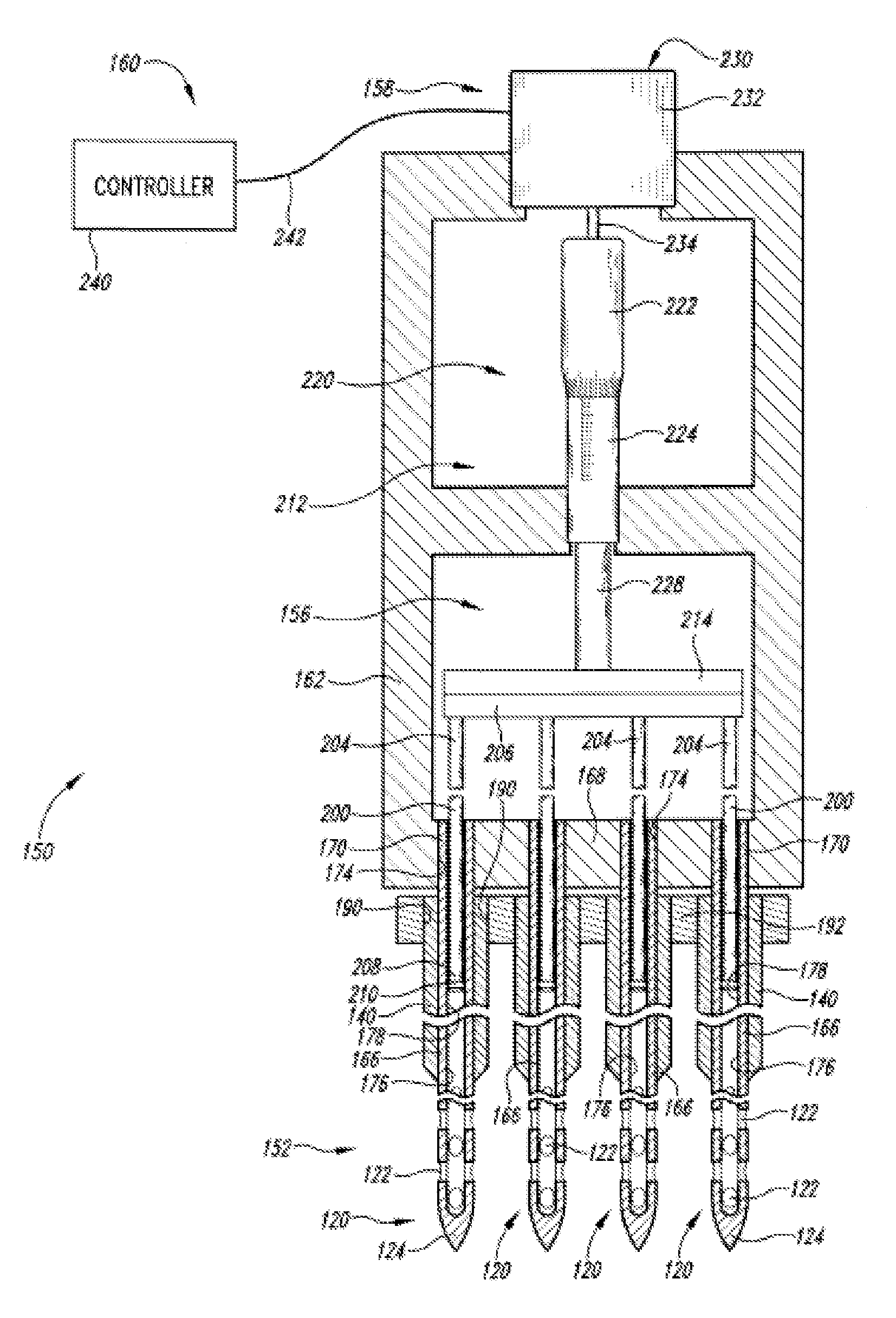
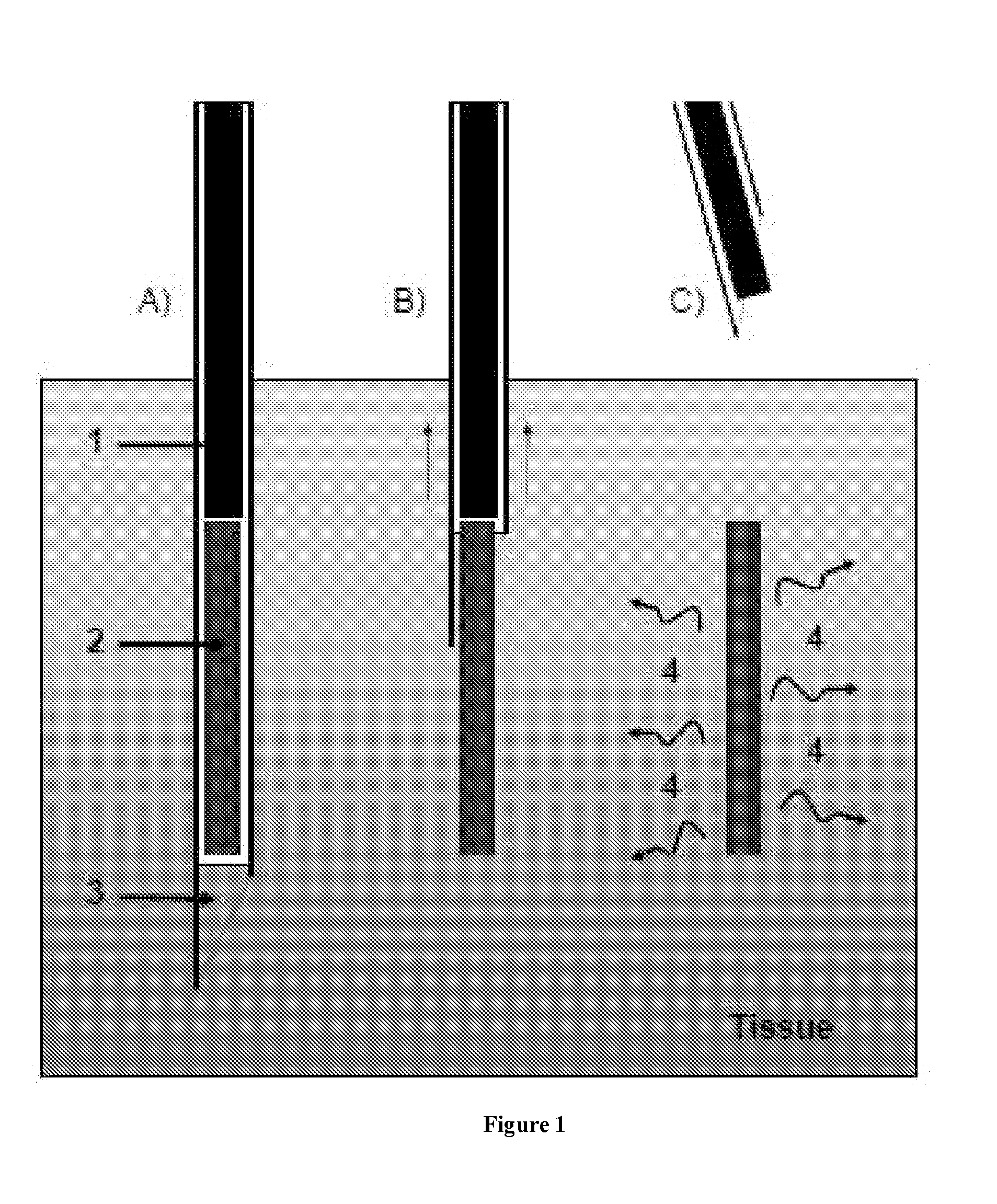
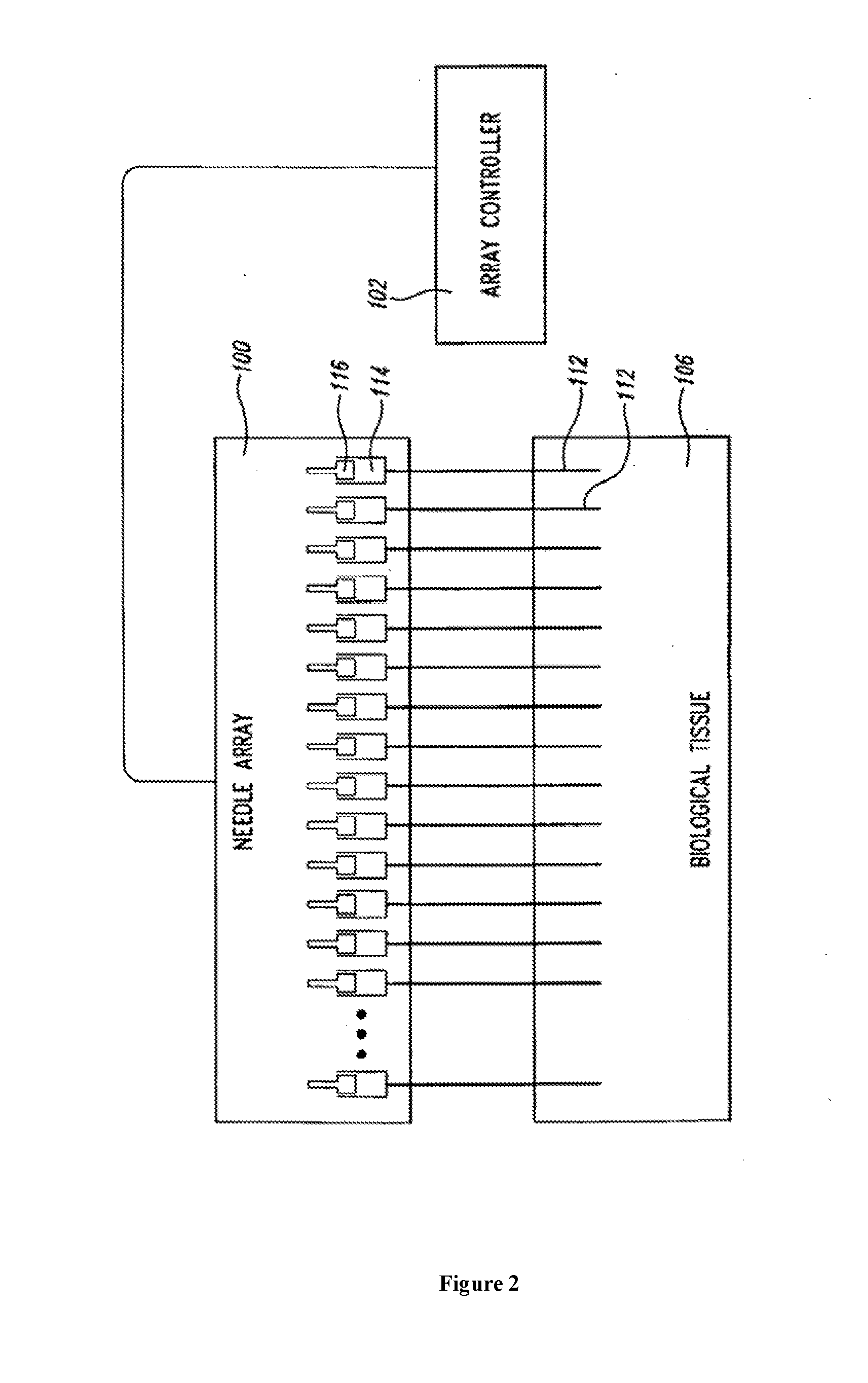
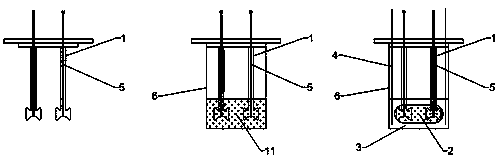
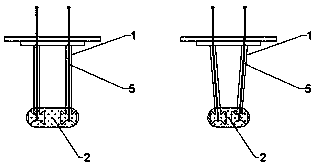
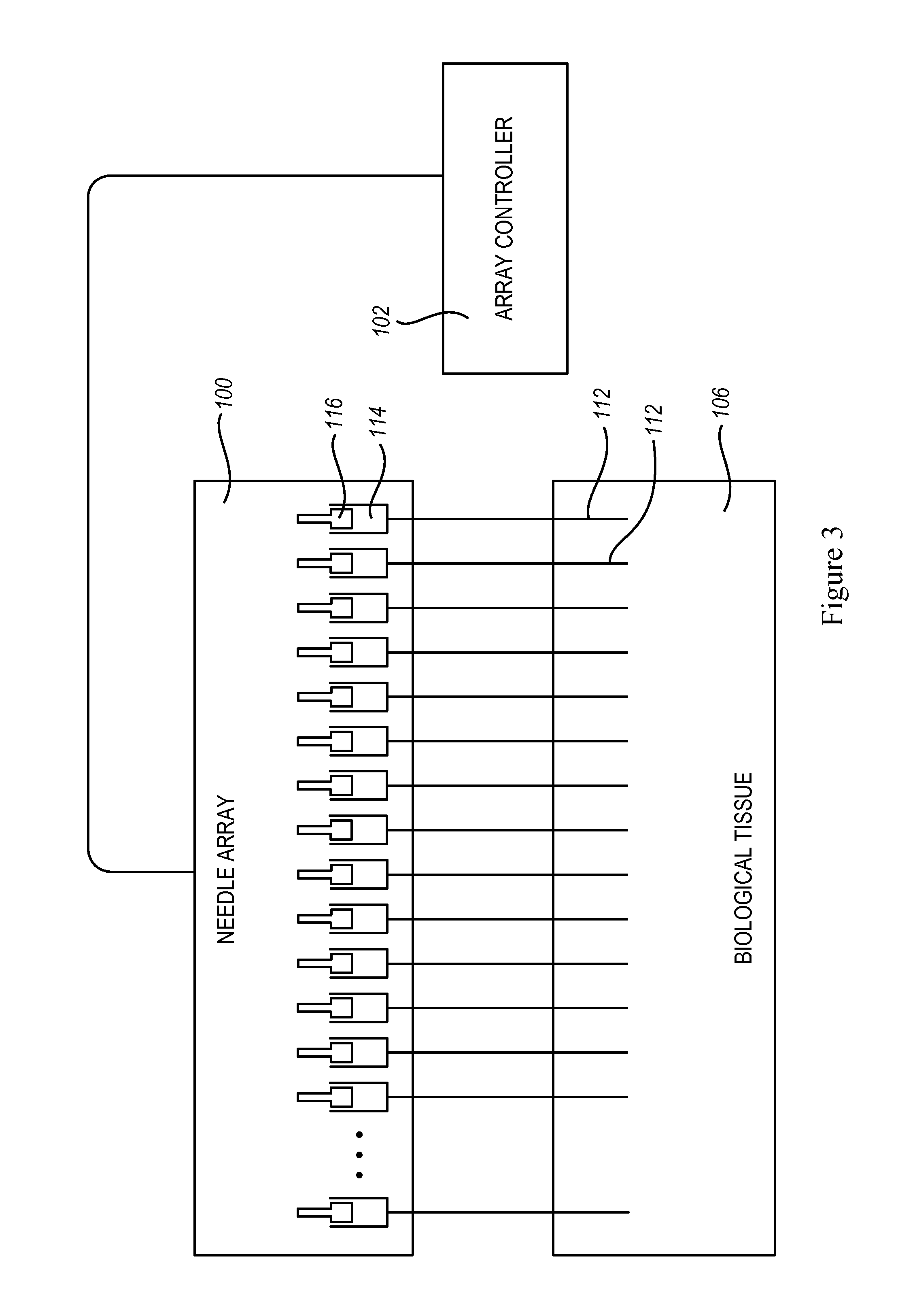
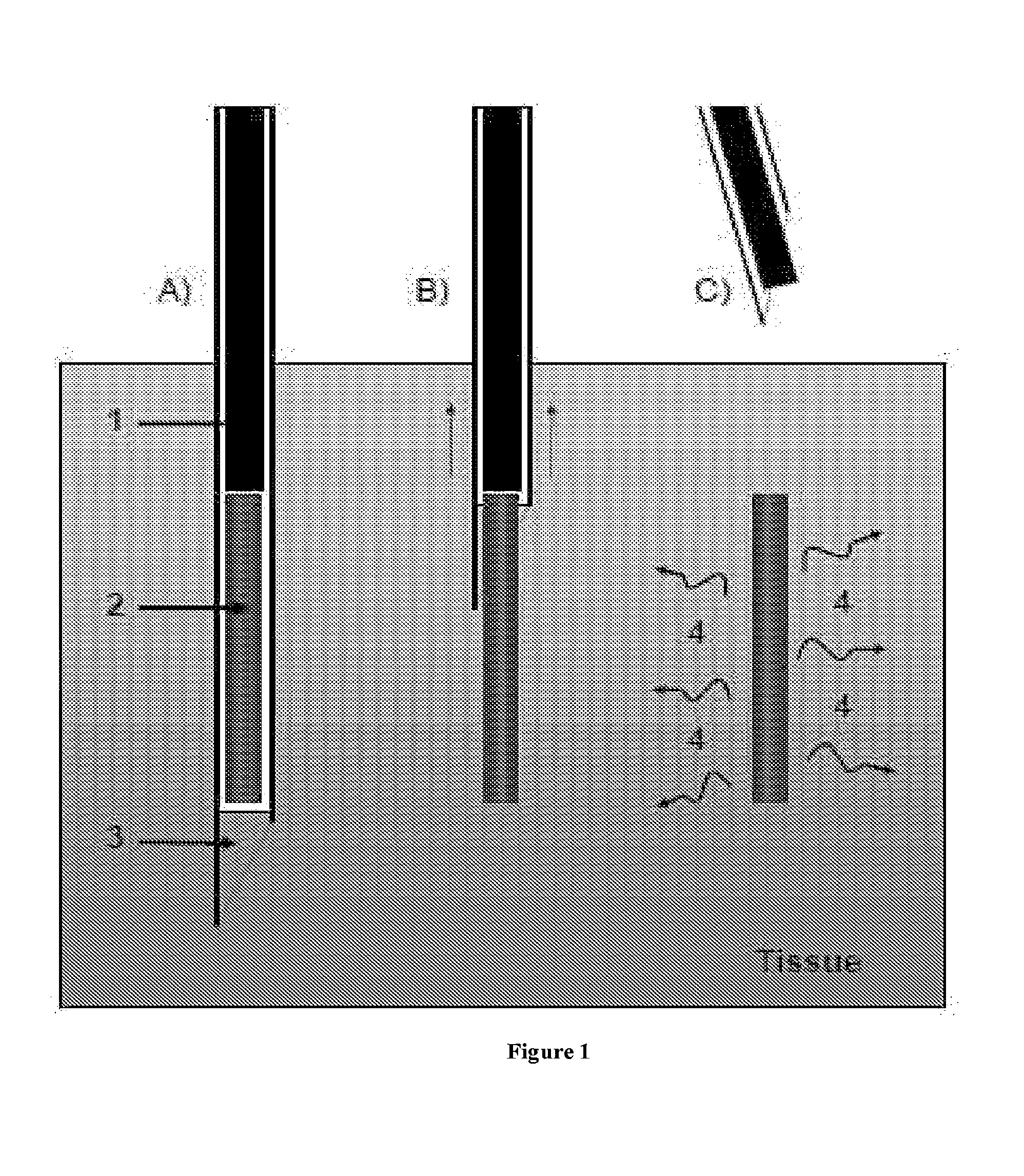
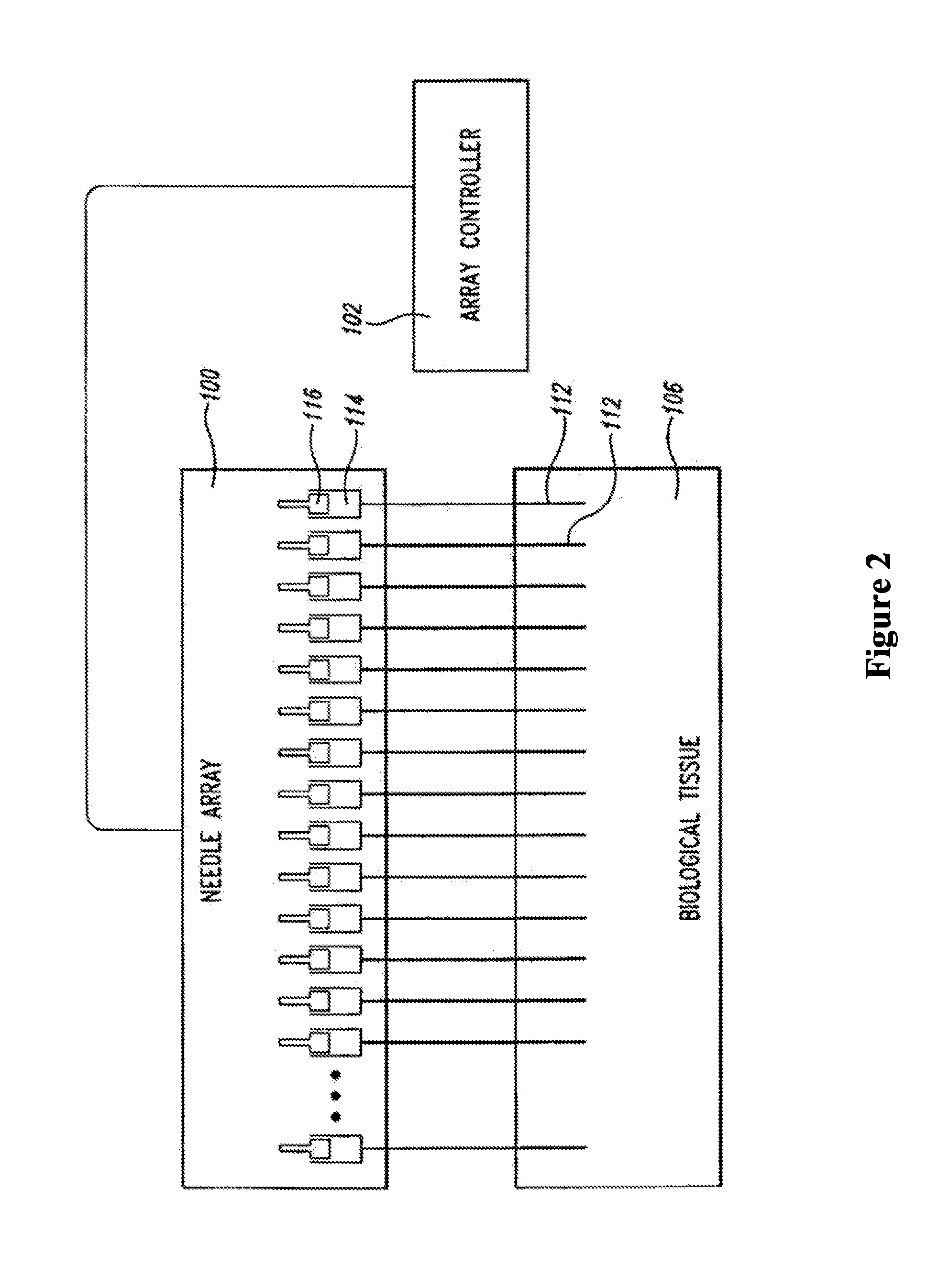
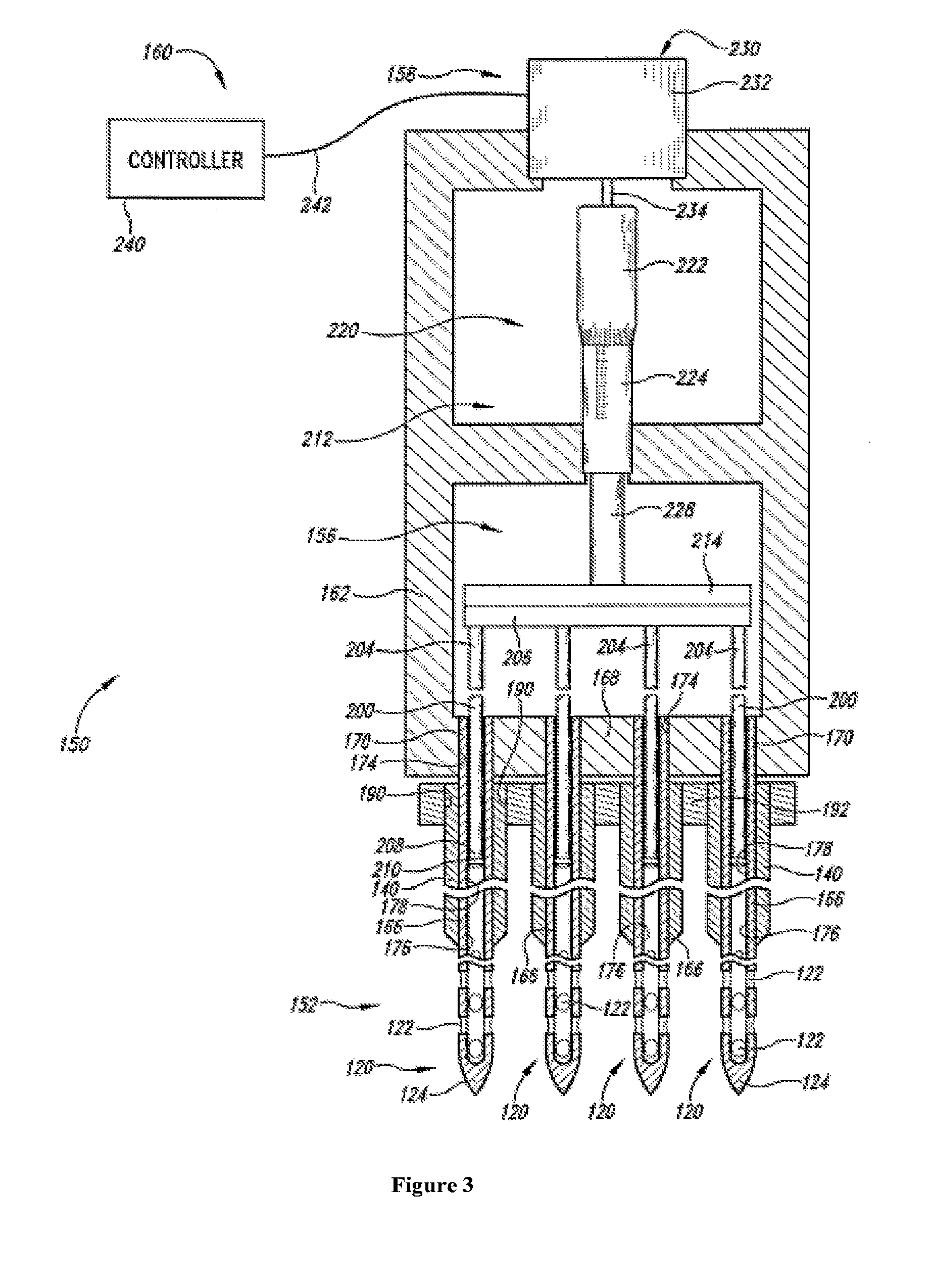
An administration device comprising an array of needles, one or more fluid agents, and at least one hydrogel is described. The device can simultaneously deliver a plurality of fluid agents along respective axes into a tissue. The use of hydrogel leads to constrained delivery of the fluid agents. The constrained delivery of an agent is also achieved by depositing a drug implant into a tissue. The effect of an agent on the tissue can be evaluated thereafter. In addition, the invention is directed to treating muscle diseases by delivering a therapeutic agent in vivo, and the use of reporter tissues for candidate drug evaluation, detecting and characterizing resistance.
Owner:PRESAGE BIOSCI
Human albumin animal models for drug evaluation, toxicology and immunogenicity studies
InactiveUS6949691B2Improve assessmentMass productionSerum albuminDrug compositionsImmunogenicity StudyCarcinogen
An animal model is provided which is genetically engineered to express human serum albumin, and such animals may be advantageously used in assessing drugs, vaccines or other therapeutic compounds that may be used in humans. In addition, an animal model is provided which does not manufacture its own albumin and which has been injected with human serum albumin. Through the use of these animal models, drugs and other chemicals can be more accurately assessed in physiological environments that reflect the conditions to be expected in humans, and such models will be useful in assessing new drugs and evaluating toxic substances for potential dangers as carcinogens, mutagens, etc. Other applications include evaluating immunological properties of various albumin-engineered proteins which might be administered to humans as therapeutics or vaccines, and research of disease states, such as genetic diseases, to provide further insight in treating these diseases.
Owner:NEW CENTURY PHARMA INC
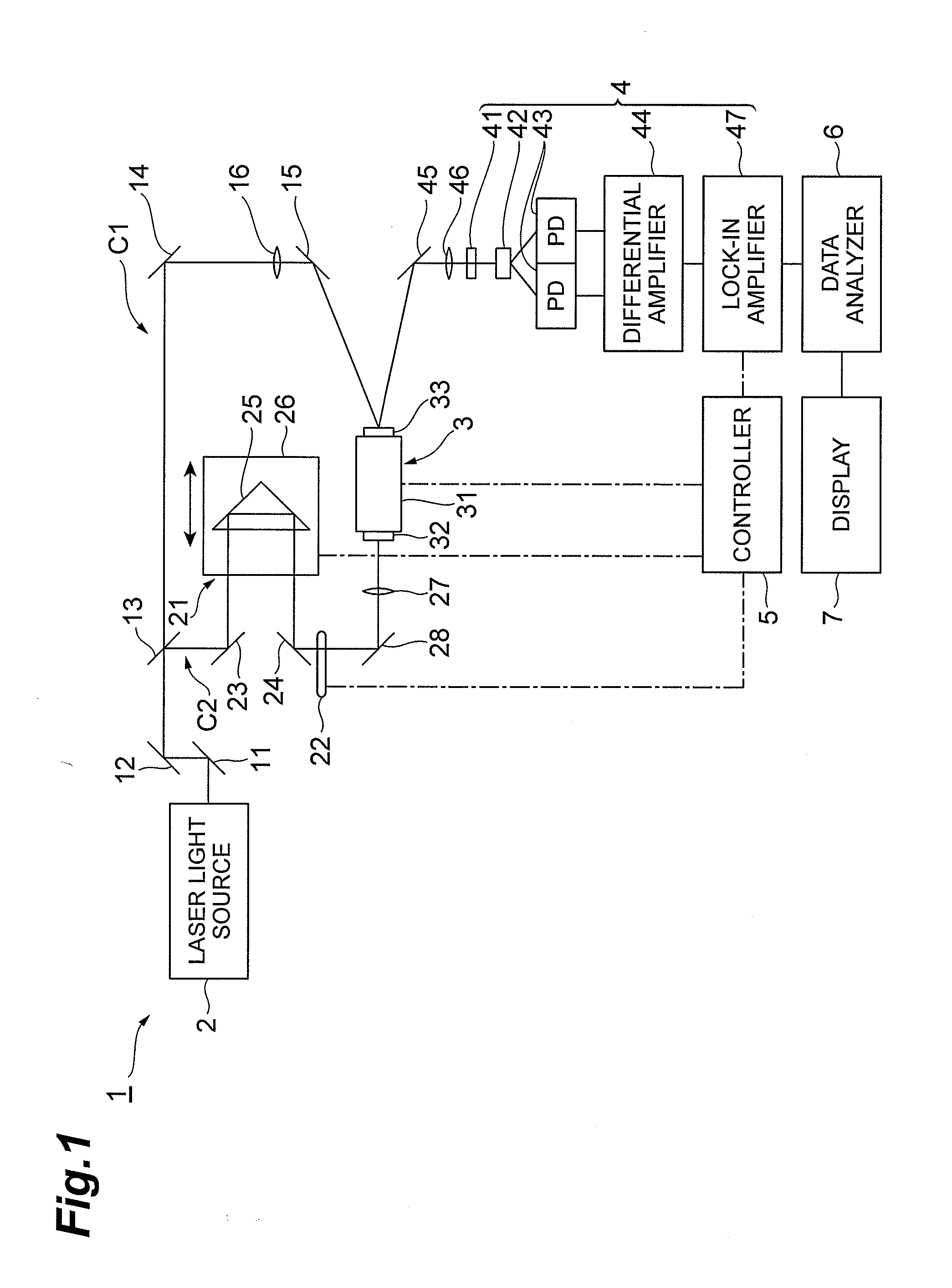
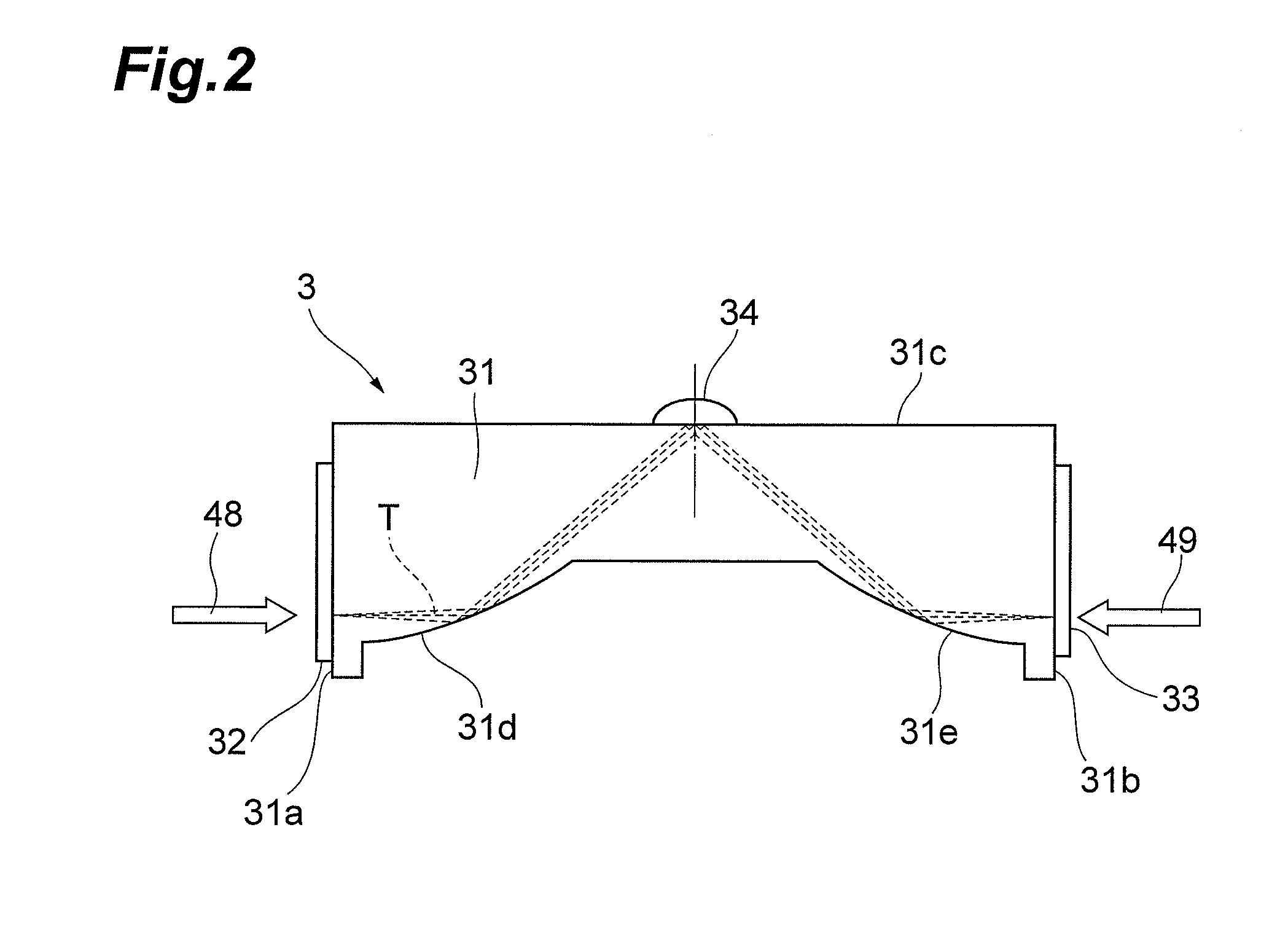
Drug evaluation method and drug evaluation device
ActiveUS20130187050A1Sure easyRadiation pyrometryColor/spectral properties measurementsMedicinePeak area
The drug evaluation device obtains, by an attenuated reflection method using a terahertz wave, an evaluation absorption spectrum for a frequency with respect to a liquid to be evaluated. When crystalline particles are suspended in a liquid, an absorption peak having a peak area corresponding to the amount of suspension appears in its absorption spectrum. Therefore, whether or not and by what ratio crystalline particles are suspended in the liquid can be determined according to whether or not the absorption peak exists and the peak area. When amorphous particles are suspended in the liquid, the baseline of its absorption spectrum lowers according to the ratio of amorphous particles suspended in the liquid. Therefore, whether or not and by what ratio amorphous particles are suspended in the liquid can be determined according to the lowering amount of the baseline.
Owner:HAMAMATSU PHOTONICS KK
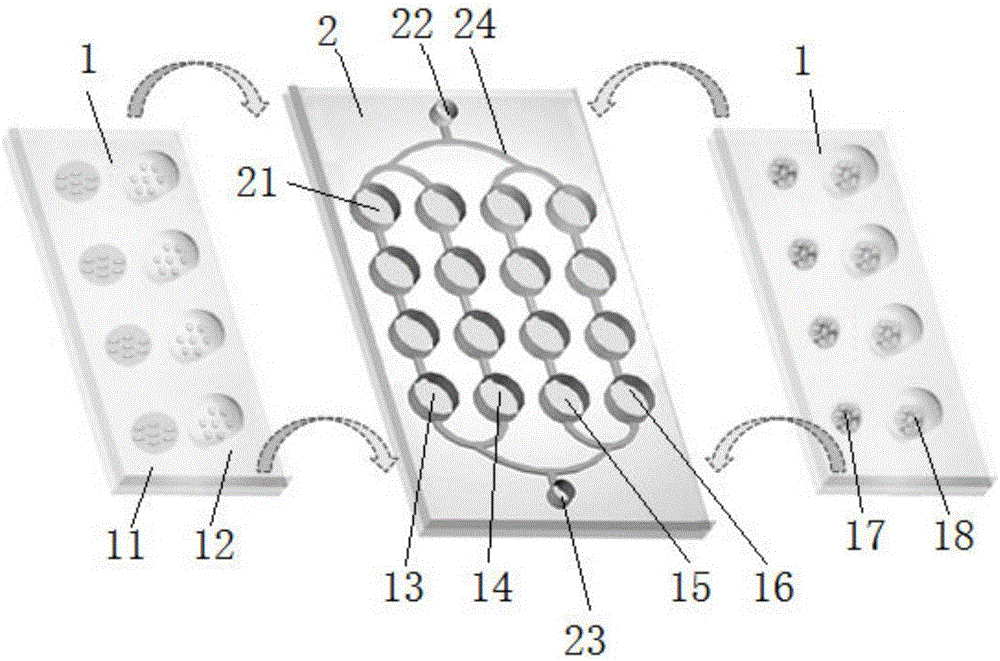
Assembly type multi-condition parallel-culture microfluidic control device and using method thereof
ActiveCN105907641AImprove recycling difficultiesOptimizationTissue/virus culture apparatusBiochemical engineeringMicrofluidic channel
The invention provides an assembly type multi-condition parallel-culture microfluidic control device which is composed of a microfluidic control chip and a cover plate, wherein the two parts can be flexibly combined, and are conveniently dismounted; the microfluidic control chip comprises a cell culture room array, a liquor inlet tank, a waste liquor tank and a microfluidic channel; the cell culture room array, the liquor inlet tank and the waste liquor tank communicate with one another through the microfluidic channel; and the cover plate detachably covers the cell culture room array. The microfluidic control device is strong in universality, is convenient to operate, and has a wide application prospect in the fields of cell treatment, tumor drug evaluation, and the like.
Owner:DALIAN NUOYI BIOTECH CO LTD
Cell array structural body and cell array
InactiveUS20080227664A1Easy and secured isolationEasy to operateBiochemistry apparatusMicroorganism librariesDrugs solutionCulture mediums
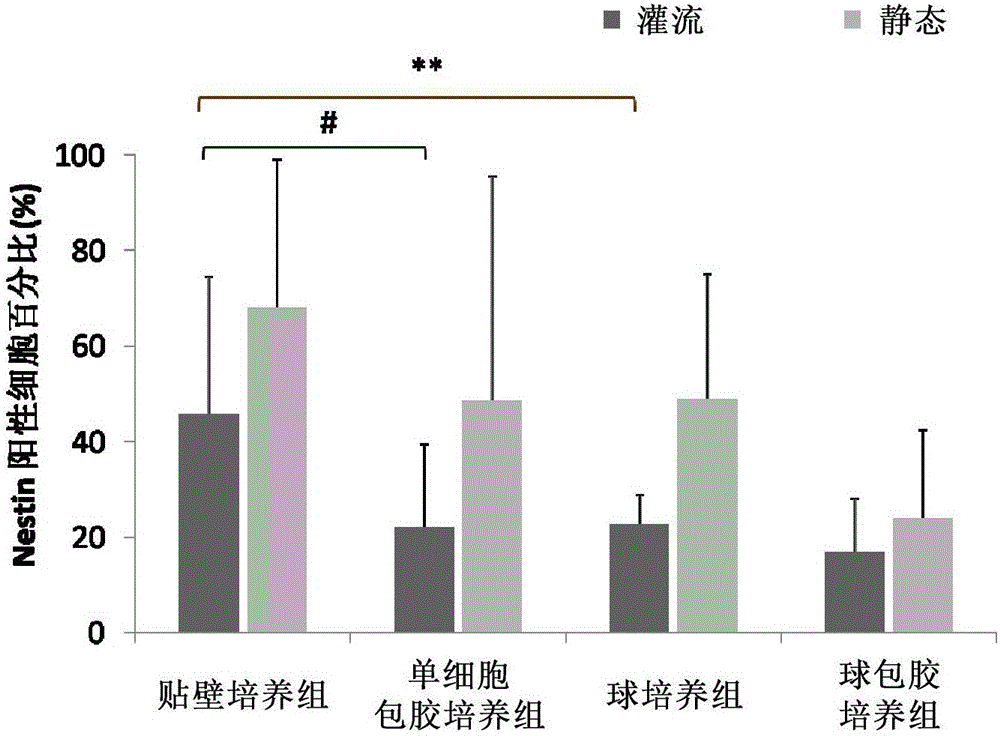
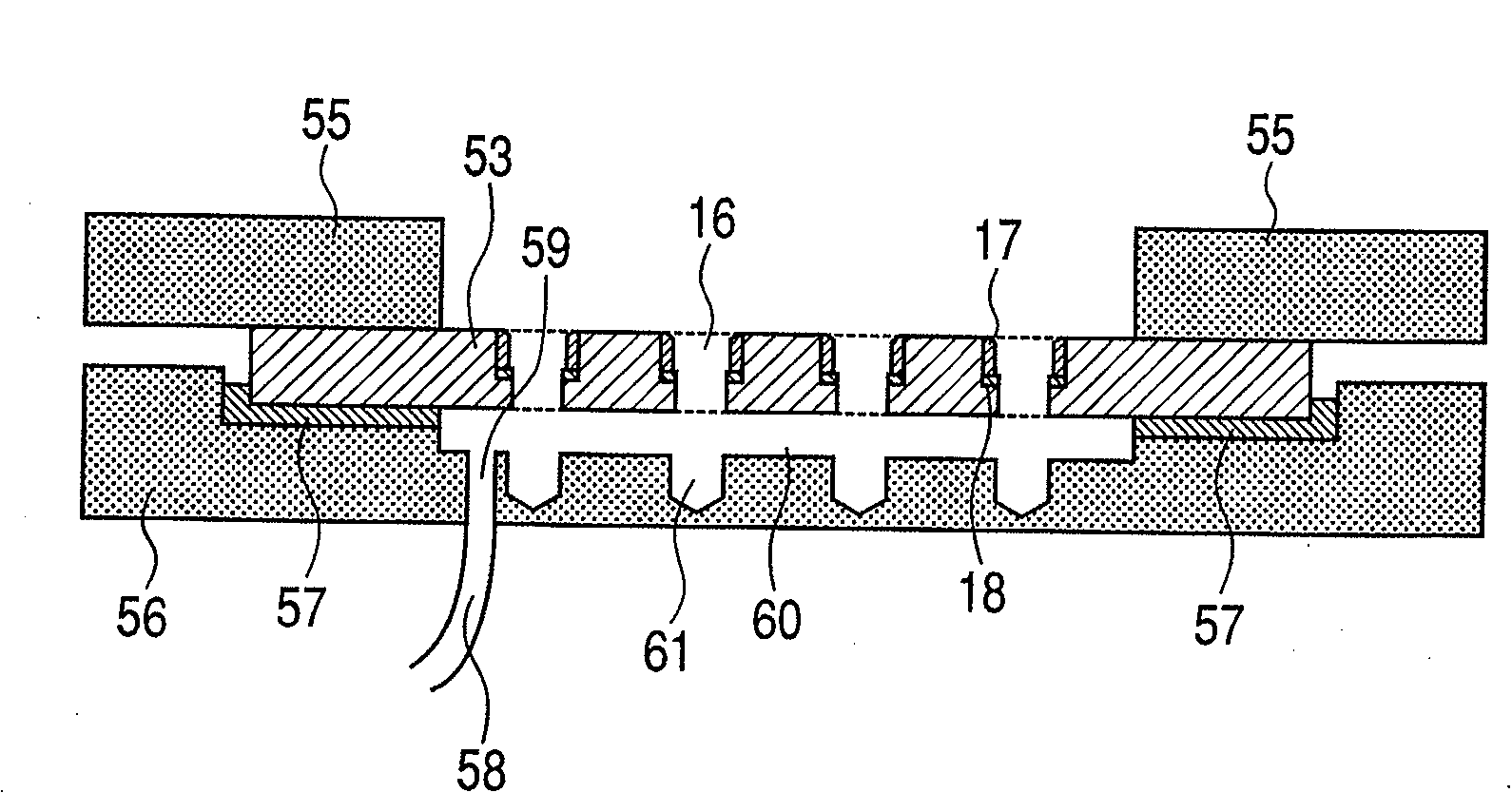
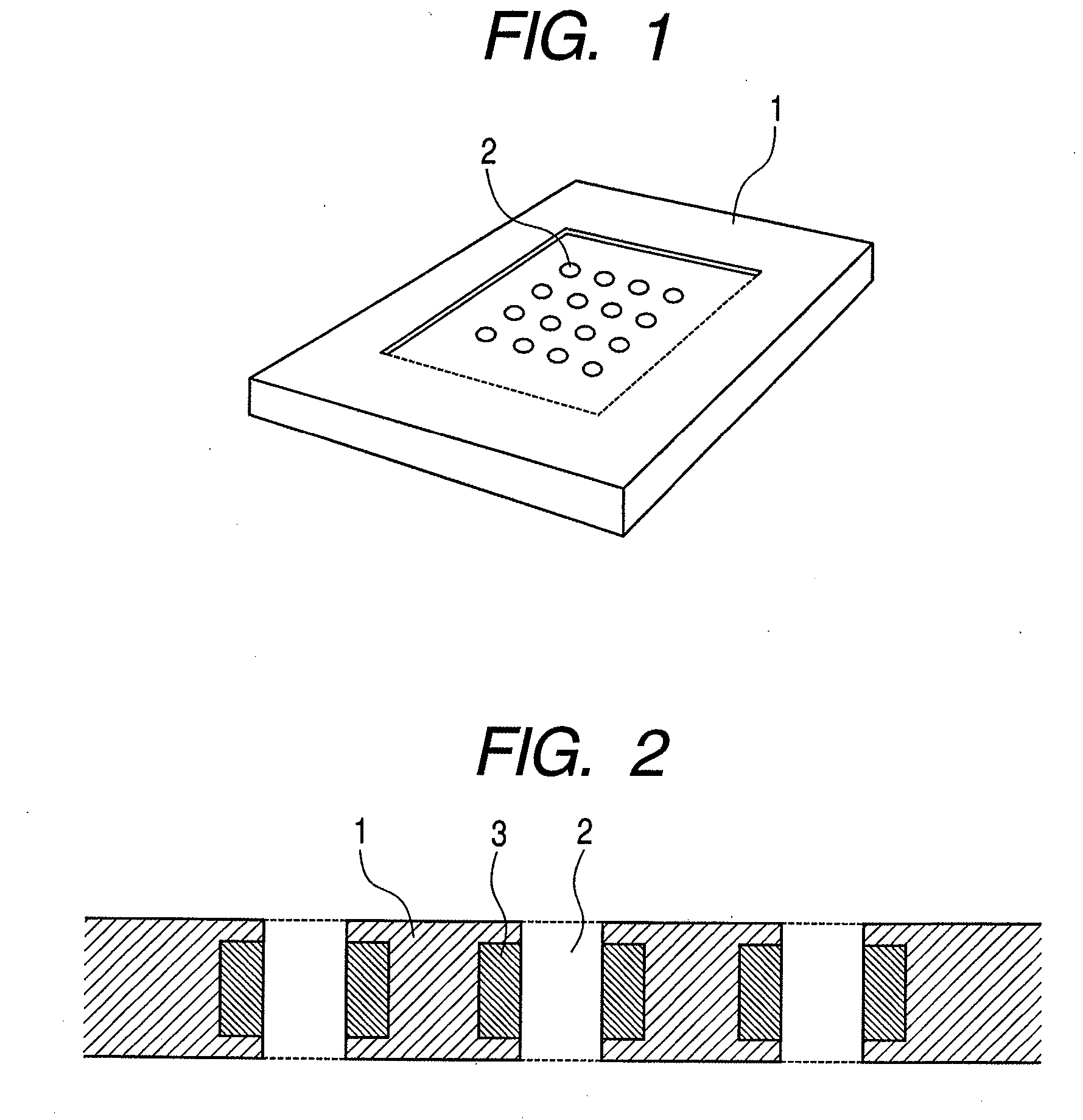
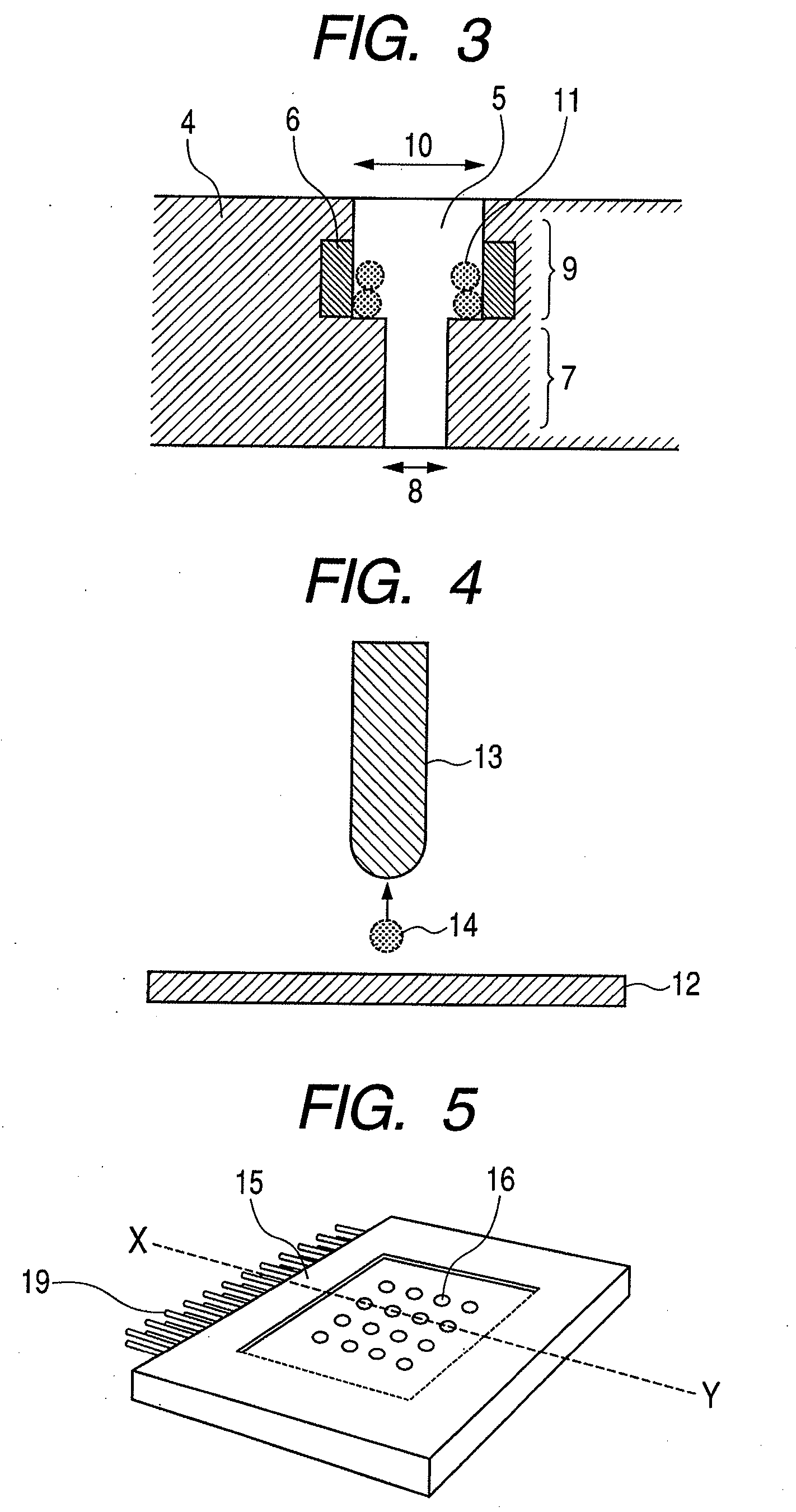
The present invention provides a cell array structural body containing a substrate, a plurality of micropores piercing the substrate from one surface to another surface, through which a sample cell can pass, and a capture / release unit for the sample cell on a wall surface of each micropore, as well as a cell array with such structural body detachably immobilizing sample cells in its micropores. In using the cell array structural body and the cell array for drug evaluations or the like, handling of a culture medium or a drug solution, or washing procedure is easy, and harvest of a desired cell from the cell array is easy and trustworthy.
Owner:CANON KK
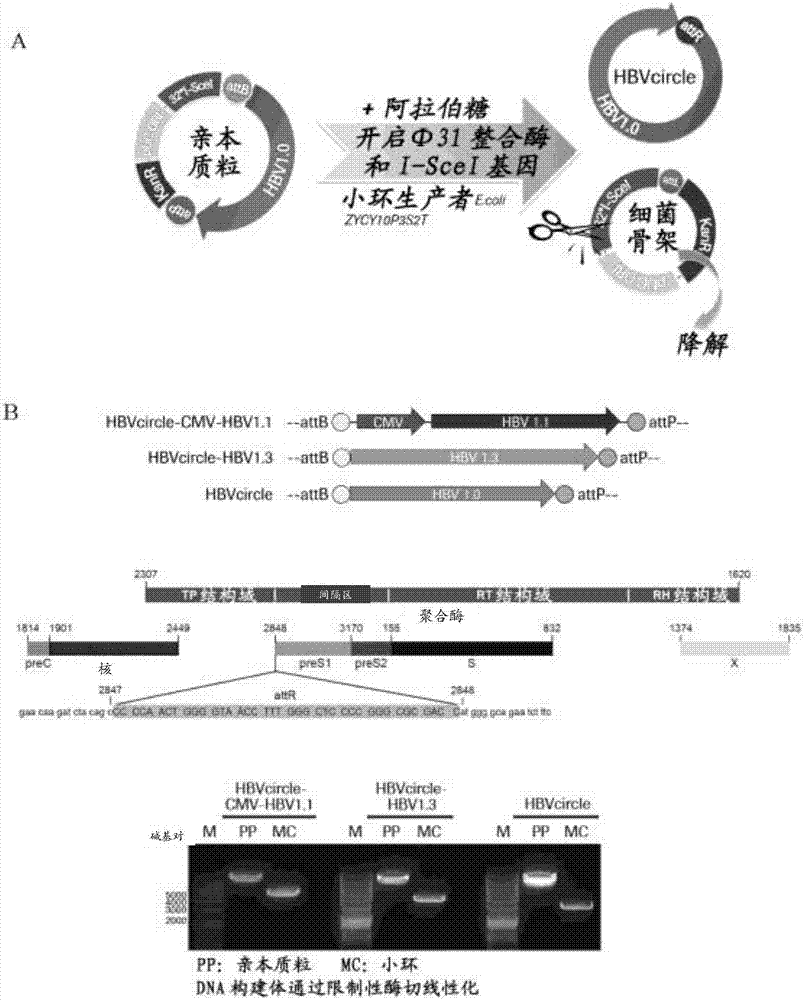
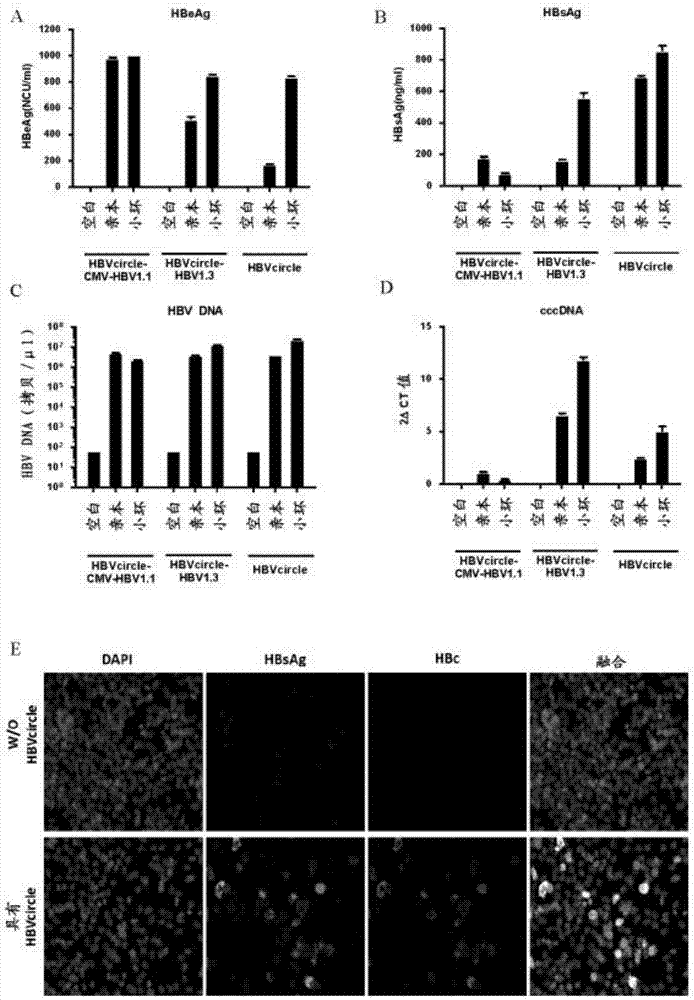
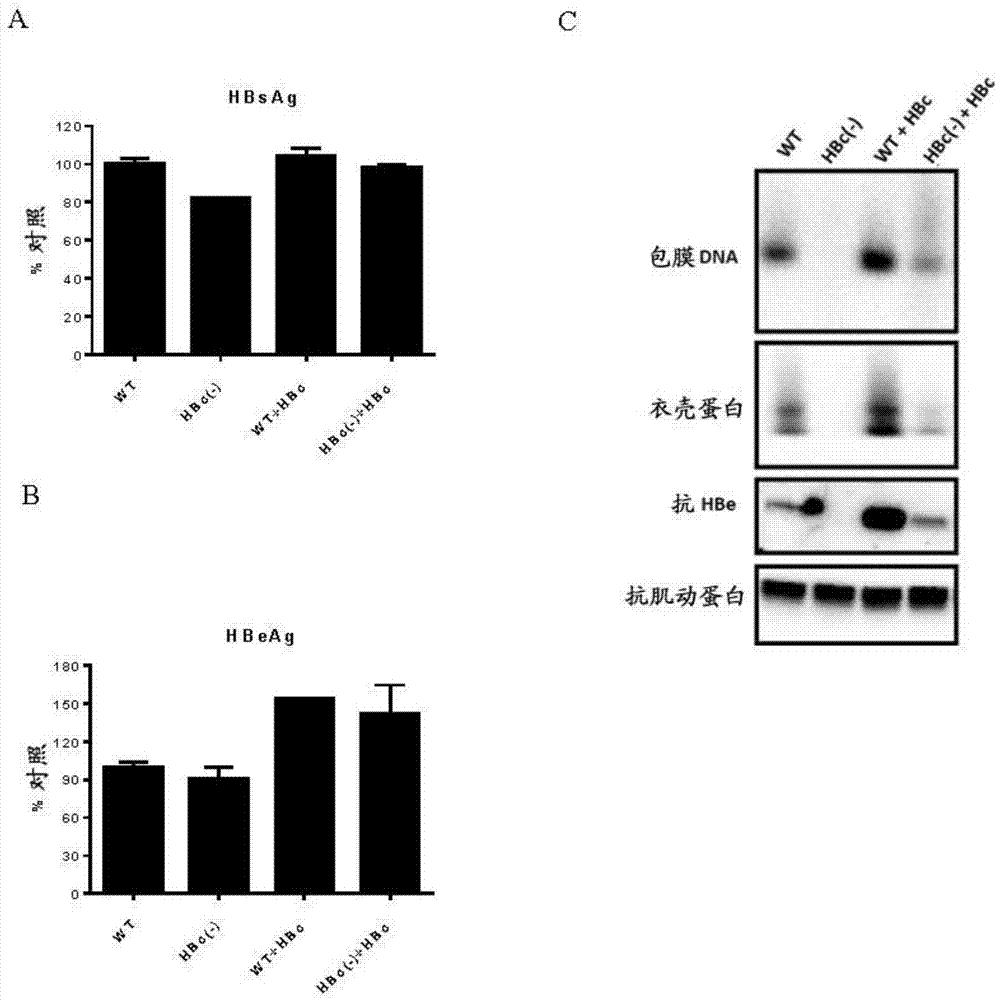
Recombinant hbv cccdna, the method to generate thereof and the use thereof
The present invention relates to a recombinant HBV cccDNA comprising HBV genome or the fragment or variant thereof and a site-hybrid insert, a method to generate said recombinant HBV cccDNA, a method for establishment of an in vitro or in vivo cccDNA based model for persistently hepatitis B virus replication by using the recombinant HBV cccDNA of the present invention, and a method for anti-HBV drug evaluation.
Owner:F HOFFMANN LA ROCHE & CO AG
Simulation system for high temperature and high humidity environment of tropics
The present invention relates to an environmental simulation system, wherein the environmental simulation system is widely used for simulating high temperature and high humidity environments in automobile manufacturing, aviation science and technology, military products, communications equipment, and other fields, but there is no ideal technical scheme of the environmental simulation system in the prior art. The purpose of the present invention is to provide a simulation system for a high temperature and high humidity environment, and a simulation method for a high temperature and high humidity environment based on the simulation system. The simulation system of the present invention mainly comprises a cabin (1), a central control system (2), an instrumentation system (3) and a technical support system (4). With the present invention, the completely-simulated natural tropical environment conditions such as high temperature, high humidity, light intensity, noisiness and the like can be provided, wherein the controllable temperature range is 20-60 DEG C, the controllable humidity range is 50-90%, the controllable illuminance range is 2000-8000 LUX, and the controllable noisiness range is 60-95 dB; the system can be adopted to perform related researches of tropical region environment issues, and can provide efficient solutions for related drug evaluations.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Establishment and application of rhesus monkey pulmonary fibrosis model
ActiveCN105126075AEasy to operateEasy to repeatSaccharide peptide ingredientsRespiratory disorderRodent modelMedicine
The invention discloses a method for preparing an animal pulmonary fibrosis model. According to the method, bleomycin is applied to a primate, the application dose of the bleomycin is 7 mg / Kg for each time, and application is performed once for each week and totally performed twice. The invention further provides a rhesus monkey pulmonary fibrosis model prepared with the method and an application of the rhesus monkey pulmonary fibrosis model. The rhesus monkey pulmonary fibrosis model is established successfully, an operation process is simple and easy to repeat, and the model can be used for new biotechnological drug evaluation which cannot be finished by a rodent model and has bright clinical application prospects.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
In vitro 3D liver model, enterohepatic co-culture model, building methods of the models and applications of the models
PendingCN109423472AAccurately predict changes in metabolic toxicityFunction increaseHepatocytesGastrointestinal cellsMixed cellLiver toxicity
The invention relates to an in vitro 3D liver model, an enterohepatic co-culture model, building methods of the models and applications of the models, belonging to the technical field of drug evaluation. The building method of the liver model includes: a step of cell culture, namely a step of culturing HepaRG cells and human hepatic stellate cells respectively, and keeping the cells for later use;a step of cell induction, namely a step of inoculating the HepaRG cells into a culture disk to perform cell culture, after the amount of the HepaRG cells adherently grown reaches a predetermined amount, performing replacement with an induction medium to perform induction culture to obtain induced HepaRG cells, and keeping the induced HepaRG cells for later use; a step of model building, namely astep of preparing a mixed cell suspension from the induced HepaRG cells and the human hepatic stellate cells, inoculating the mixed cell suspension to a preset carrier, and performing culturing to obtain a micro-tissue having a hepatic globule three-dimensional structure, that is the 3D liver model. The liver model that is the micro-tissue having a hepatic globule three-dimensional structure can well simulate actual physiological condition of the liver in the body, thereby accurately predicting liver toxicity of a drug.
Owner:NAT INST FOR FOOD & DRUG CONTROL
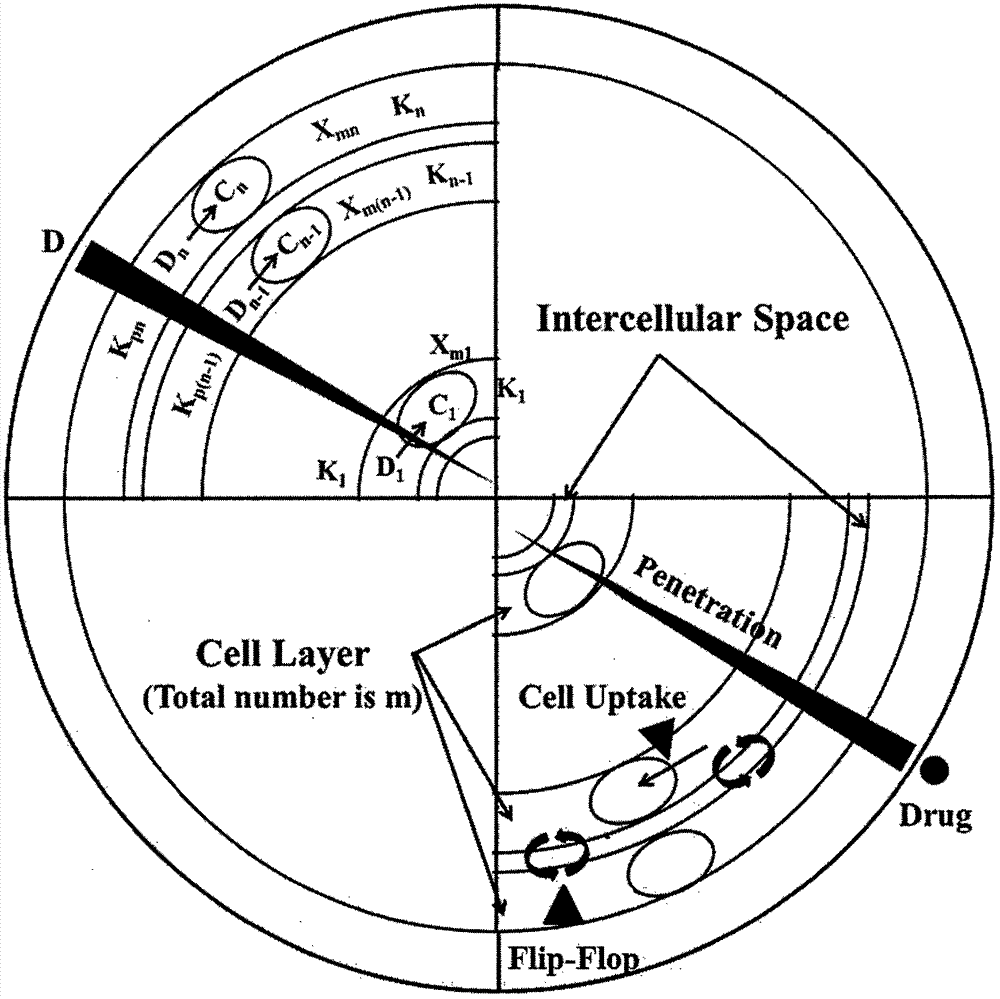
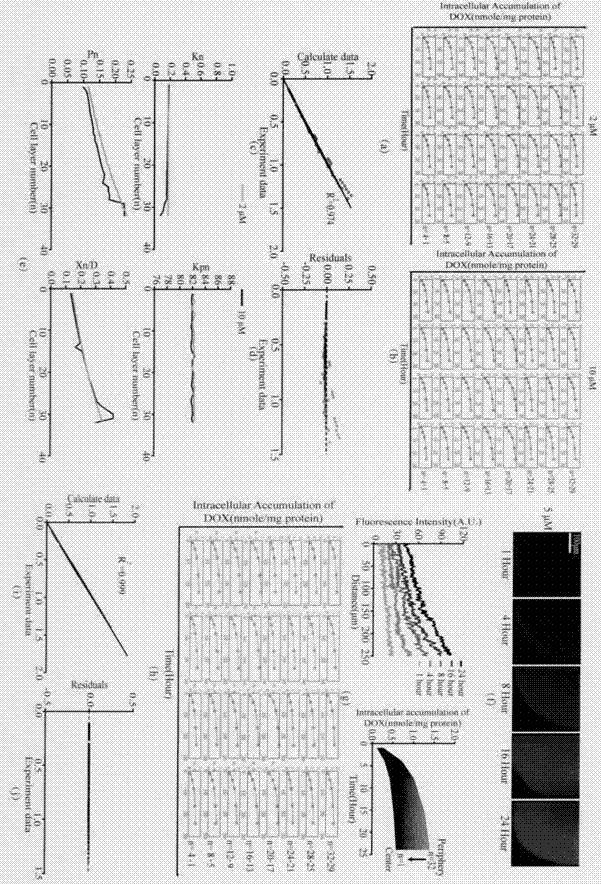
Construction of drug penetration dynamic model based on three-dimensional cell model and application of drug penetration dynamic model to drug evaluation
The invention creatively discloses a drug penetration dynamic evaluation method based on a three-dimensional cell model. The method comprises the steps that (1) a three-dimensional somatic cell model and a "penetration dynamics" mathematic model are established, the penetration behavior of an antitumor drug in somatic cells and an accumulation process in all layers of cells are described semi-quantitatively, and fitting is performed to obtain corresponding dynamic parameters; (2) drug concentrations in all layers of cells at different time points after the drug is given according to different concentrations can be subjected to backward prediction based on the obtained dynamic parameters, and the prediction result is highly consistent with an experience result; (3) biological factors influencing characteristic dynamic parameters are analyzed, the penetration behavior of the drug in tumor tissue is speculated according to a plasma drug concentration time course, and the speculation result is highly consistent with an experiment result; and (4) the model and the evaluation method are applied to a second-phase clinical drug INNO-206, and reasons for superiority of the clinical effect of the drug to that of doxorubicin are expounded from the aspect of in-tumor penetration, so that the universality of the method is further verified.
Owner:CHINA PHARM UNIV
Cell model for diabetes complicated with depression, and establishing method and application thereof
InactiveCN105441391AAvoid damageMicrobiological testing/measurementNervous system cellsBiotechnologyFlow cytometry technique
The invention belongs to the technical field of biotechnology and especially relates to a method for establishing a cell model for diabetes complicated with depression. The method comprises the following steps: adding glucose with a final concentration of 50 to 200 mmol / L and corticosterone with a concentration of 100 to 400 [mu]mol / L into a medium for culturing hippocampal neurons and carrying out intervention culture on the hippocampal neurons for 18 to 24 h so as to obtain the cell model. The cell model is evaluated through general morphological observation, HE dyeing, glucose consumption detection, intracellular neurotransmitter detection, MTT-process detection, flow cytometry, Hoechst fluorescent staining and TUNEL staining; and evaluation results show that the cell model has good effect and can be used for pathological and physiological research on diabetes complicated with depression, research on pathogenesis, in-vitro drug screening or drug evaluation.
Owner:HUNAN UNIV OF CHINESE MEDICINE
Drug target prediction and drug comprehensive evaluation method
ActiveCN107083423AAccurately and comprehensively reflect authenticityLow costNucleotide librariesMicrobiological testing/measurementLiving systemsHigh dimensionality
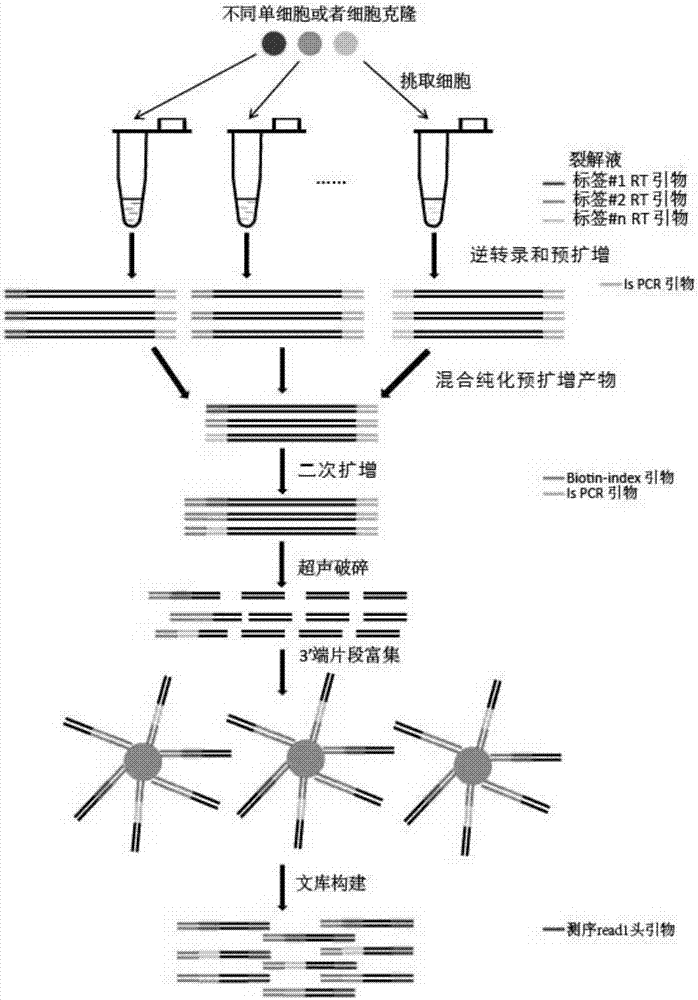
The invention provides a drug target prediction or drug evaluation method, a multi-label whole transcriptome sequencing library construction method and a multi-label whole transcriptome sequencing library constructed by the method. The method and the library of the invention are suitable for single cells or a few cells, are low-cost, have low quantity demand on samples, and can help realize high throughput accurate evaluation of the action of a drug in a living system. The invention relates to a new generation high-accuracy and high-dimensionality drug screening and evaluation system capable of comprehensive systematic research on the mechanism of drug action and can predict potential combination effect among different drugs and possible new drug research and development target so as to guide new drug research and development and clinical administration.
Owner:北京极客基因科技有限公司
Method and system for estimating correlation of gene sequence and pharmacological reaction of medicament
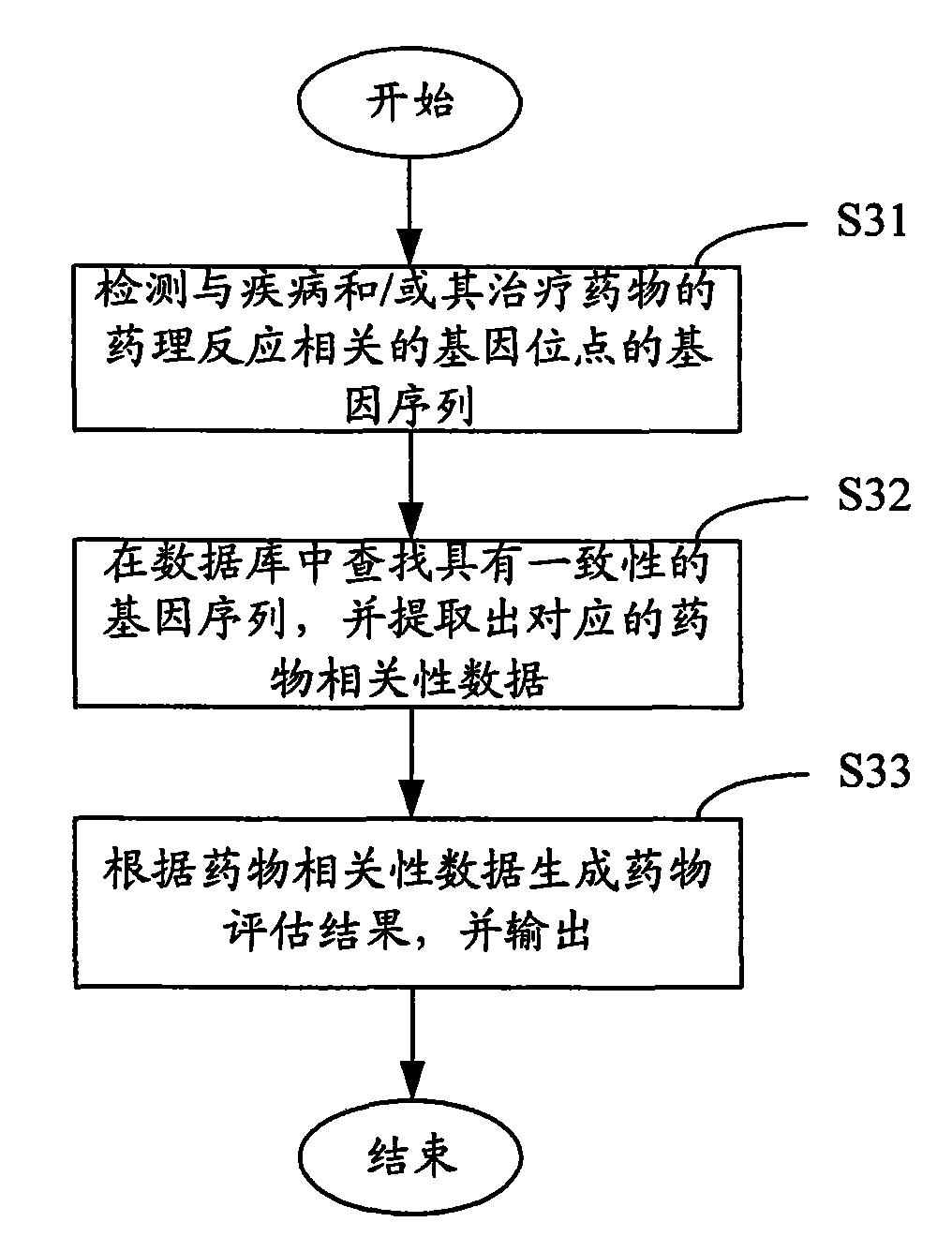
InactiveCN101603088AClear correlationImprove targetingMicrobiological testing/measurementSpecial data processing applicationsGene engineeringDisease cause
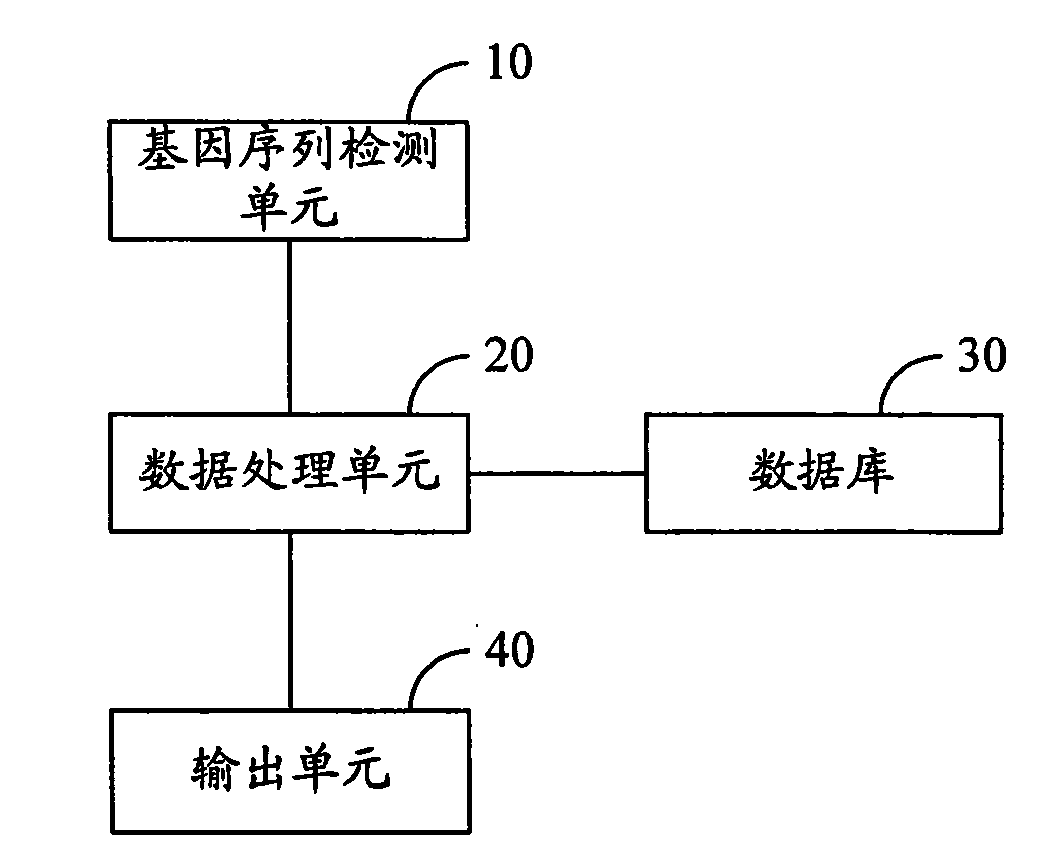
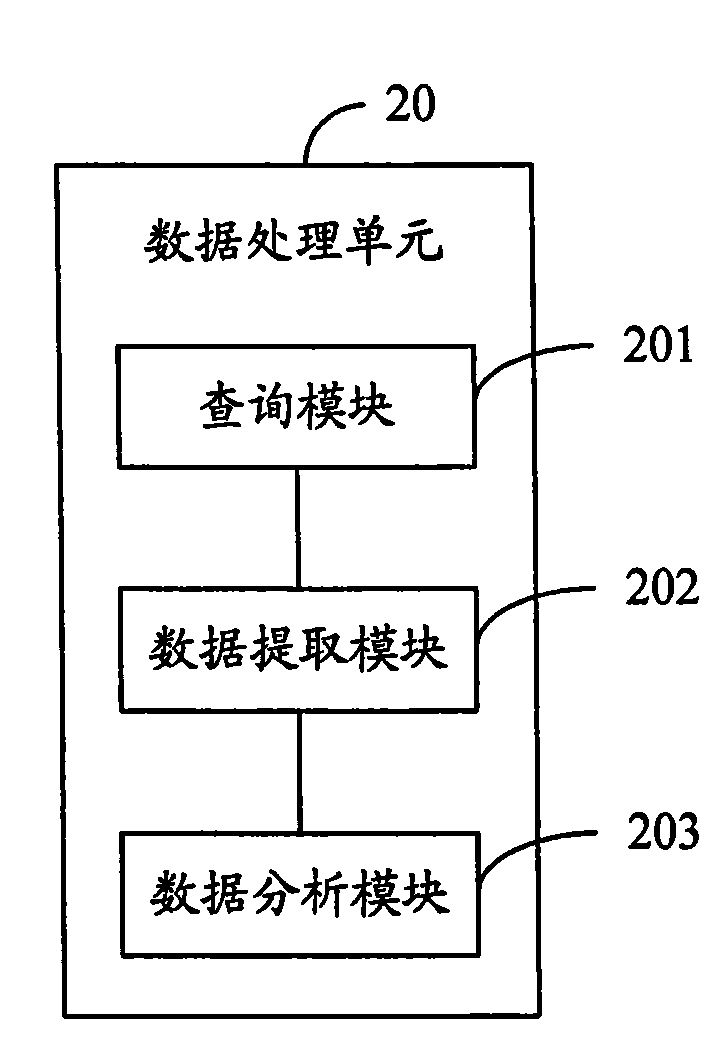
The invention relates to the fields of gene engineering and biomedical engineering, and provides a method and a system for estimating correlation of gene sequence and pharmacological reaction of a medicament. The method comprises the following steps: A, detecting the gene sequence of gene locus related with a disease and / or the pharmacological reaction of the medicament for treating the disease; B, searching gene sequence which is consistent with the detected gene sequence in a database, and extracting corresponding medicament correlation data; and C, generating a medicament estimation result according to the medicament correlation data, and outputting the medicament estimation result. The system comprises the database, a gene sequence detection unit, a data processing unit and an output unit. The method and the system confirm the correlation between the pharmacological reaction of the medicament and the disease so as to improve the medication pertinency in a follow-up treatment process.
Owner:张俊 +3
Construction method and application of in-vitro respiratory exposure model
ActiveCN109486746APrevent collapseMicrobiological testing/measurementEpidermal cells/skin cellsCell culture mediaToxic material
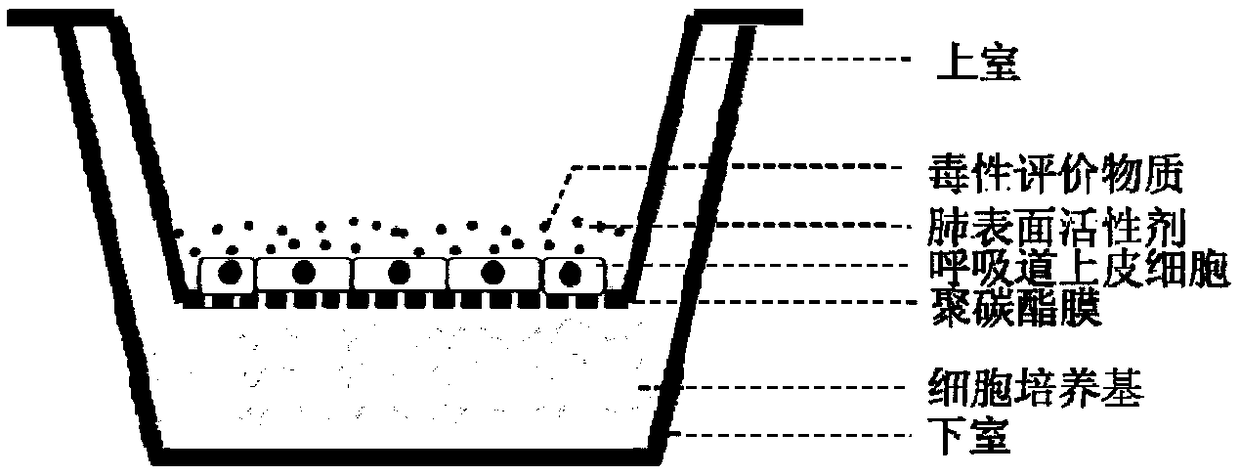
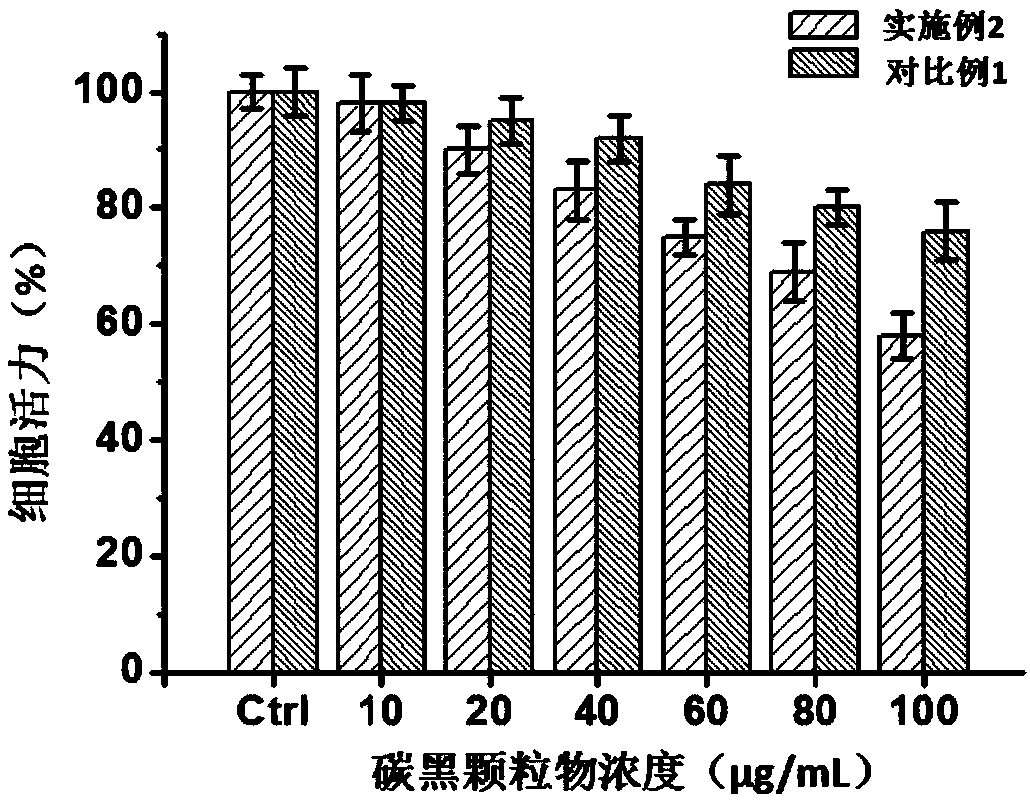
The invention provides a construction method of an in-vitro respiratory exposure model, comprising the steps of inoculating respiratory epithelial cells to a porous membrane material on a co-culture system, culturing until the cells attach to a wall, wherein the membrane material divides the co-culture system into an upper chamber and a lower chamber; adding a cell culture medium into the lower chamber of the co-culture system, adding a lung surfactant to the upper chamber, and continuing to culture for 0.5-2 hours; adding a toxic material or a drug into the upper chamber to carry out exposure, and evaluating toxicological indexes of the respiratory epithelial cells. Application of the construction method of the in-vitro respiratory exposure model in environmental pollutant or drug evaluation is also provided.
Owner:QINGDAO UNIV
Double-emitting fluorescent nanoparticle preparation method
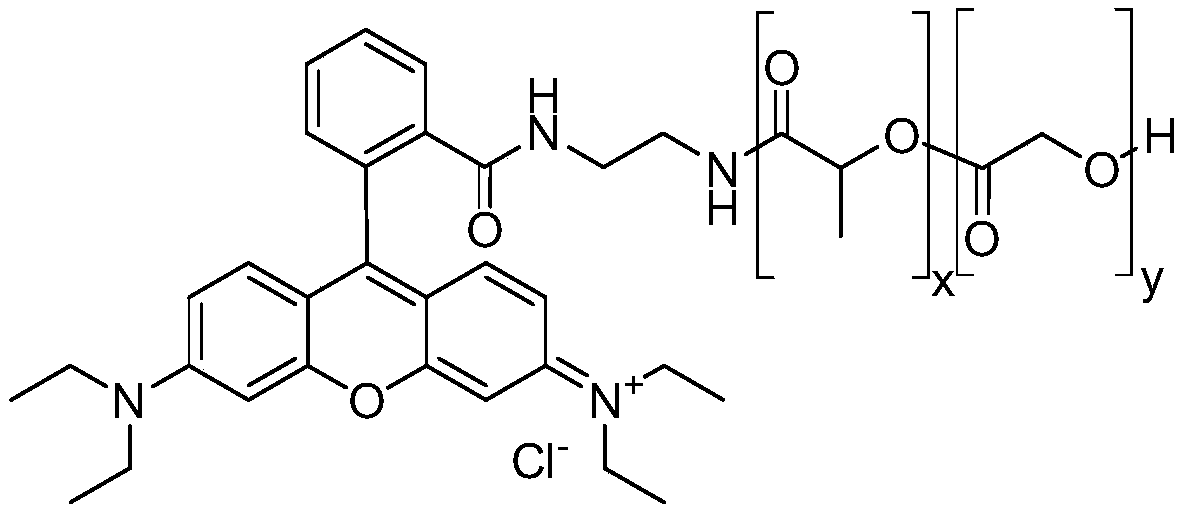
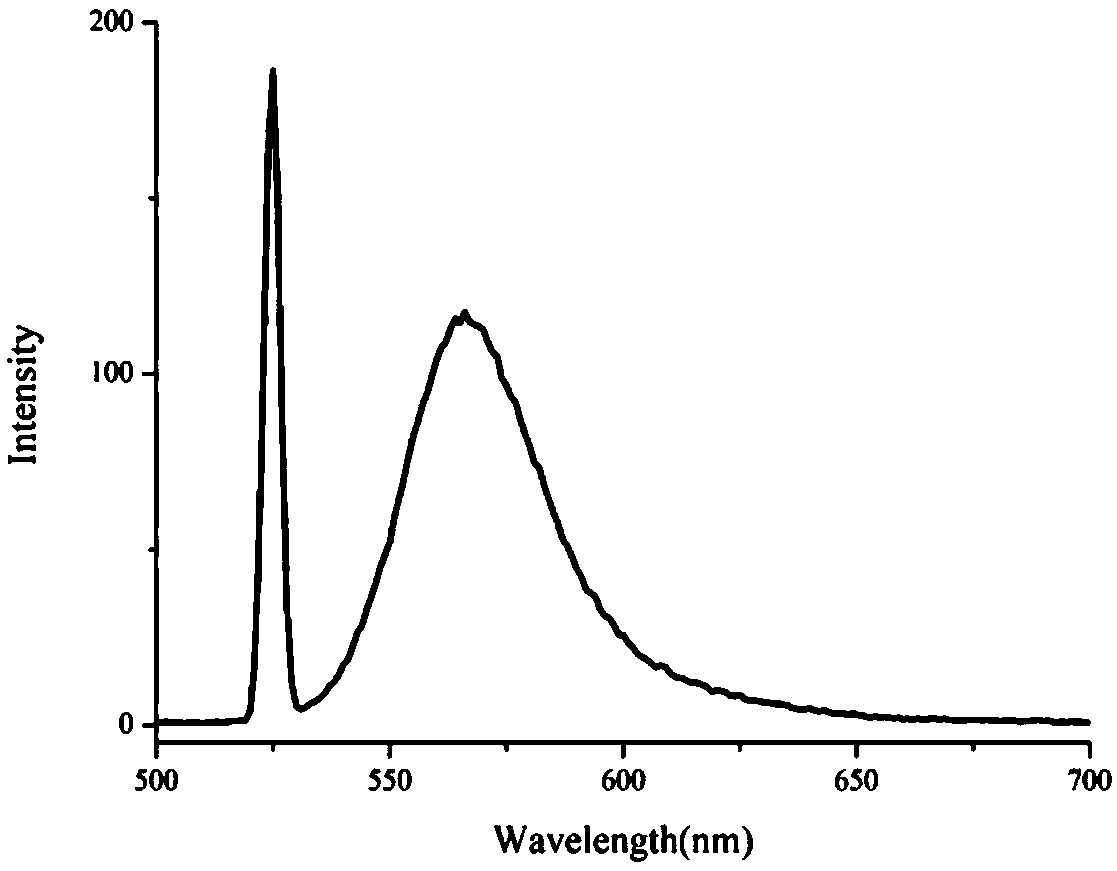
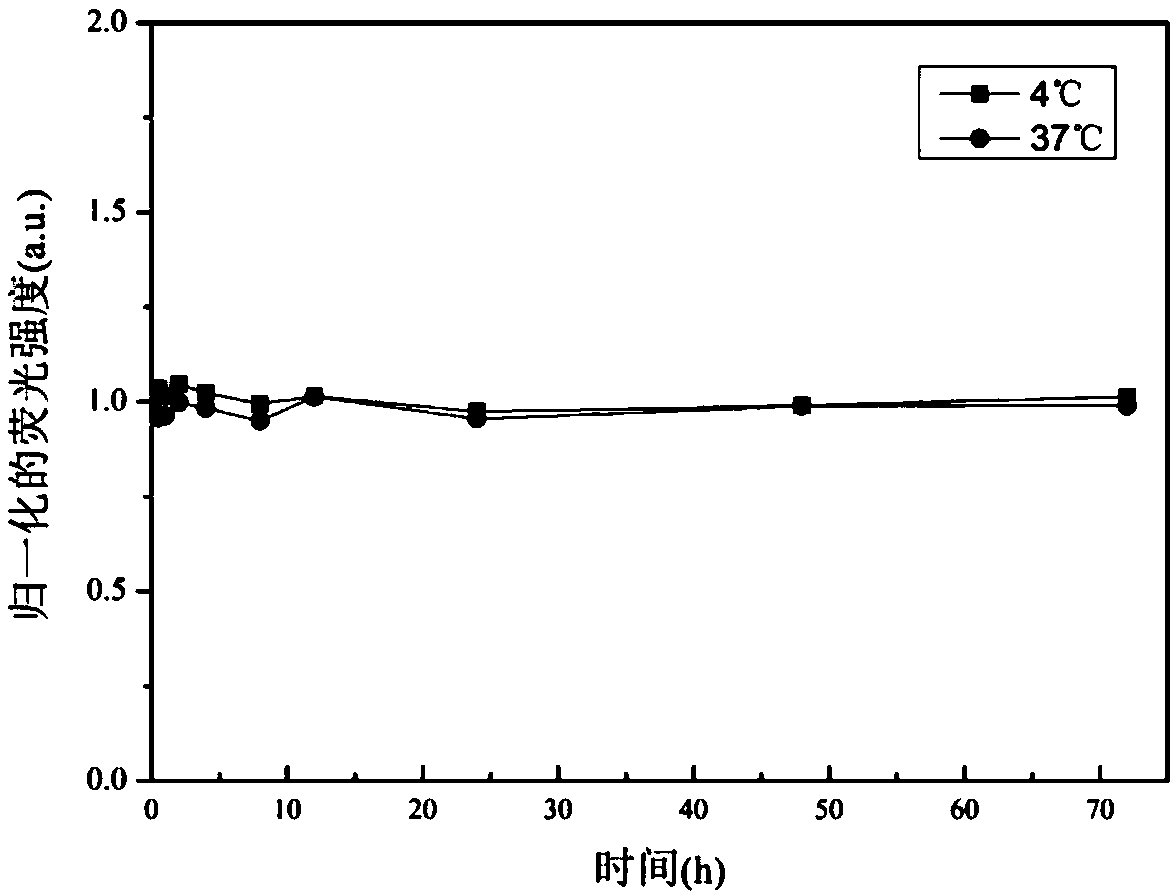
ActiveCN109265669ADoes not affect opticsDoes not affect physical and chemicalLuminescent compositionsEthylenediamineCopolymer
The invention relates to a double-emitting fluorescent nanoparticle preparation method, which comprises: covalently linking rhodamine B and a polylactic acid-glycolic acid copolymer through an intermediate linking molecule ethylenediamine to prepare a biocompatible material Rhodamine B modified polylactic acid-glycolic acid copolymer with fluorescence emission function, and encapsulating a fluorescent tracer with the fluorescent biocompatible material to obtain the double-emitting fluorescent nanoparticles. According to the present invention, the preparation method has characteristics of simple and easily-available raw materials and mild reaction conditions, and the prepared double-emitting fluorescent nanoparticles have characteristics of smooth and complete surface, uniform particle sizedistribution, good stability and good dual fluorescence emission performance, can achieve visual monitoring, can be used in the fields of clinical diagnosis and drug evaluation, and has good application prospects.
Owner:XI AN JIAOTONG UNIV
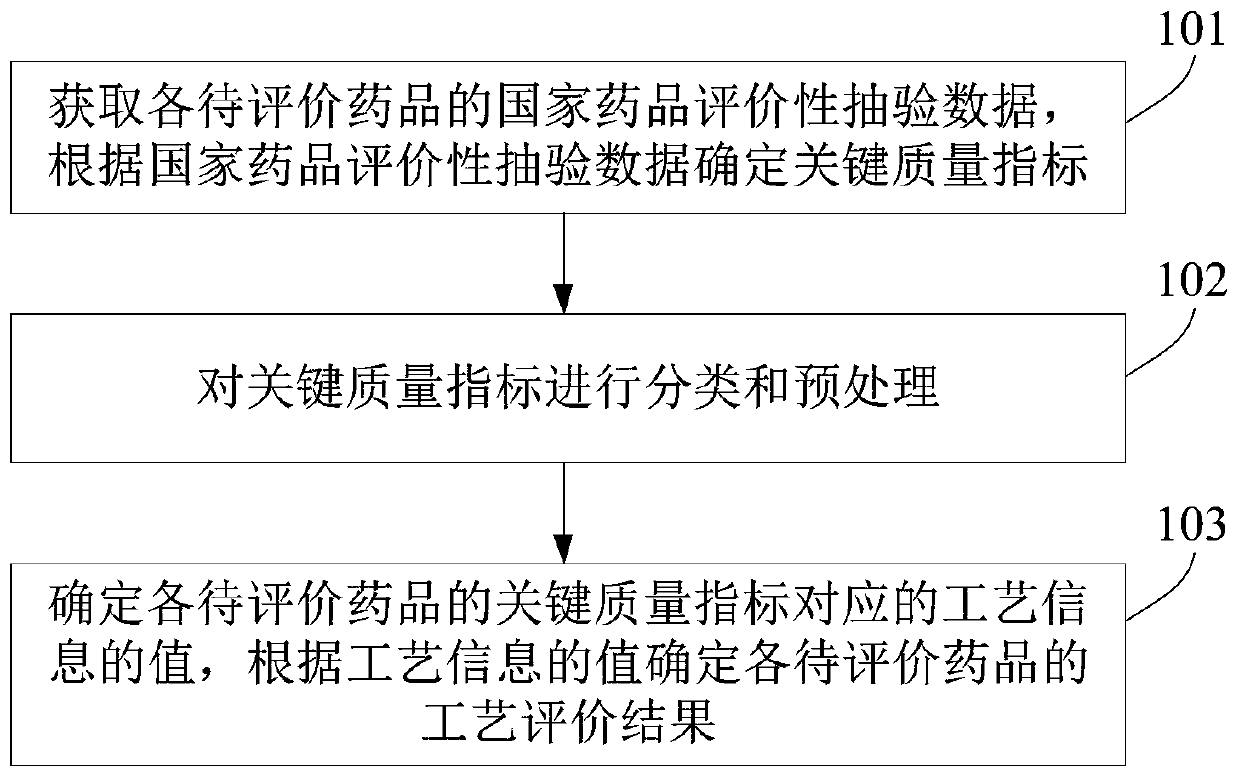
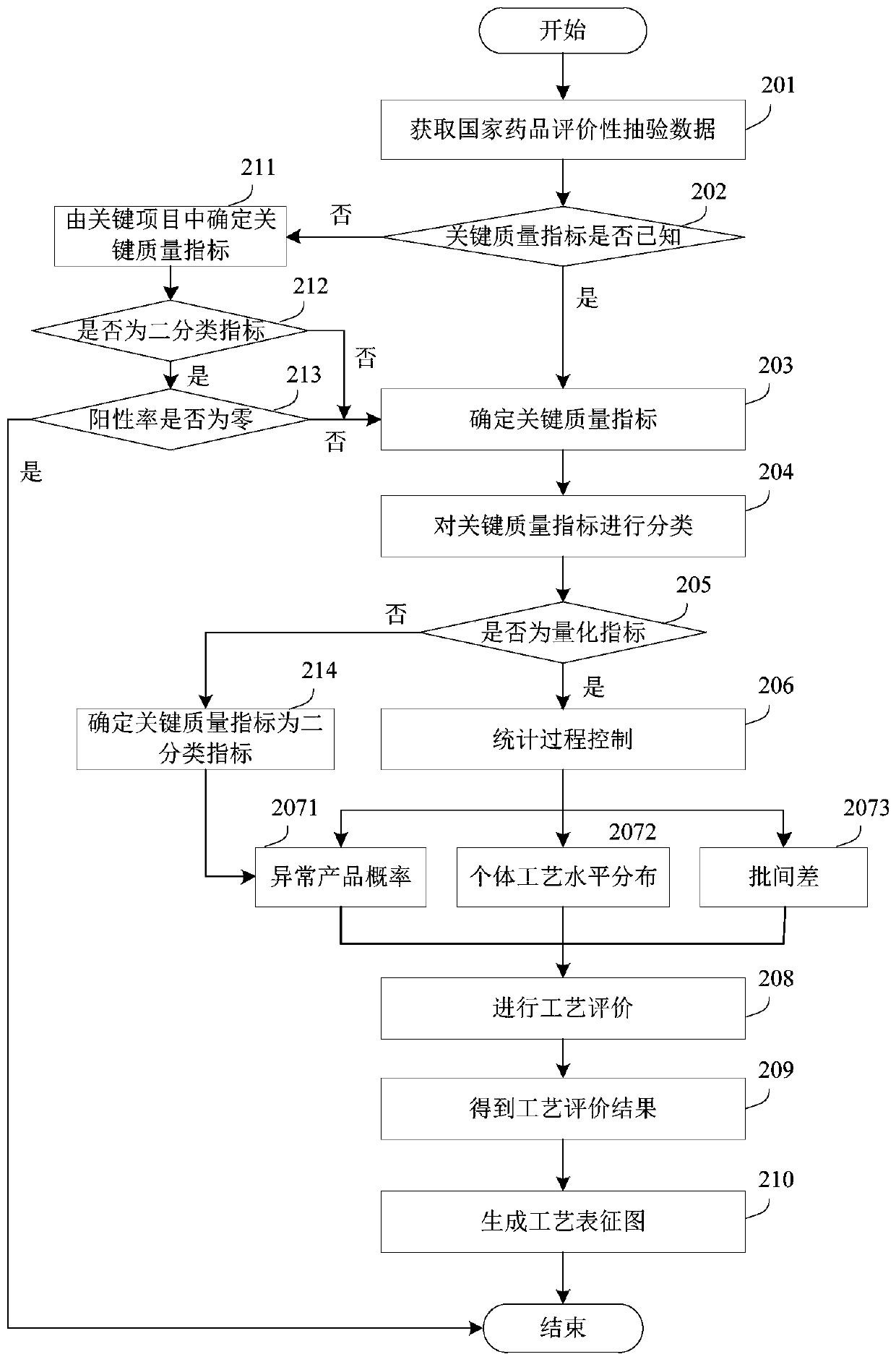
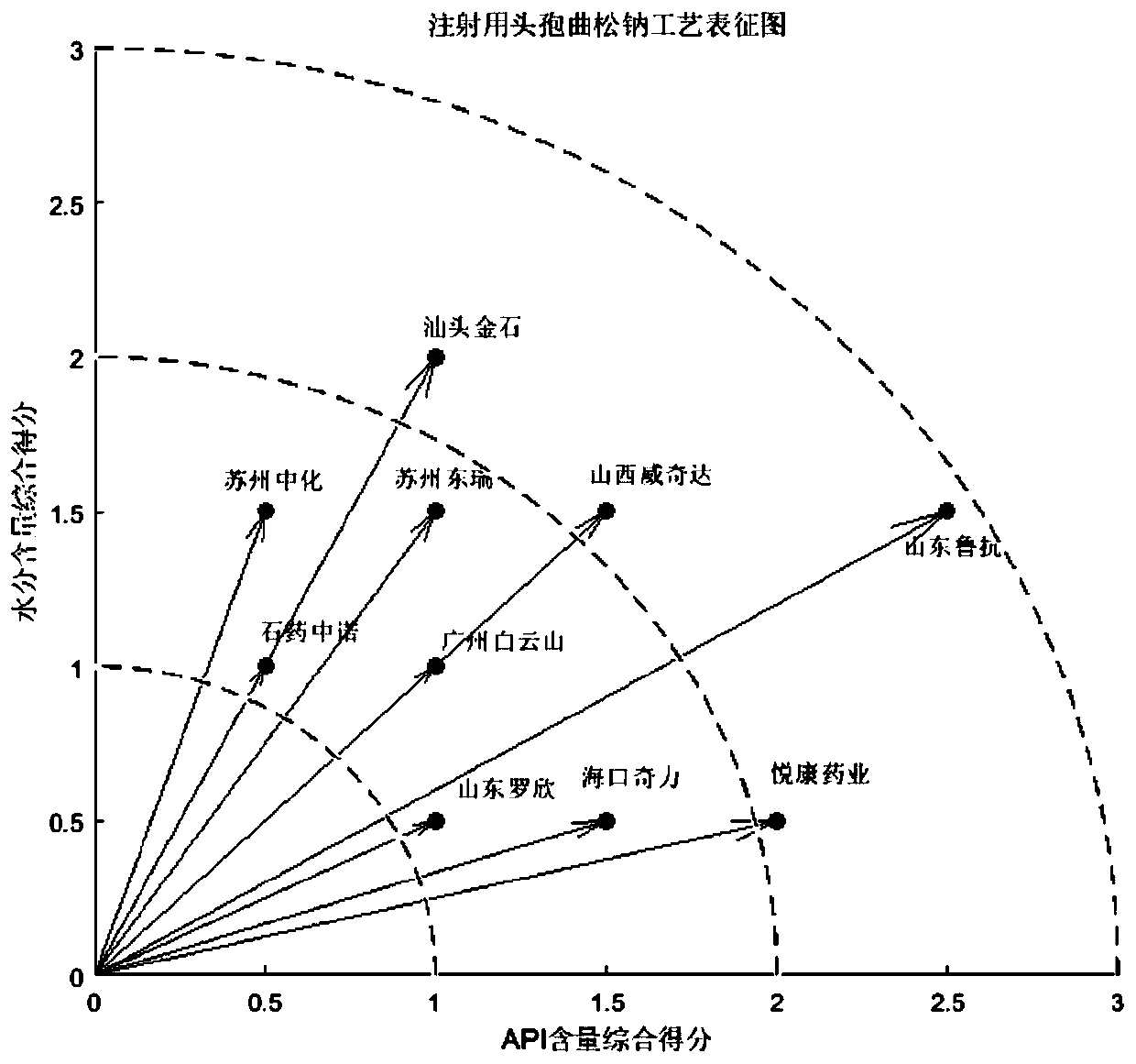
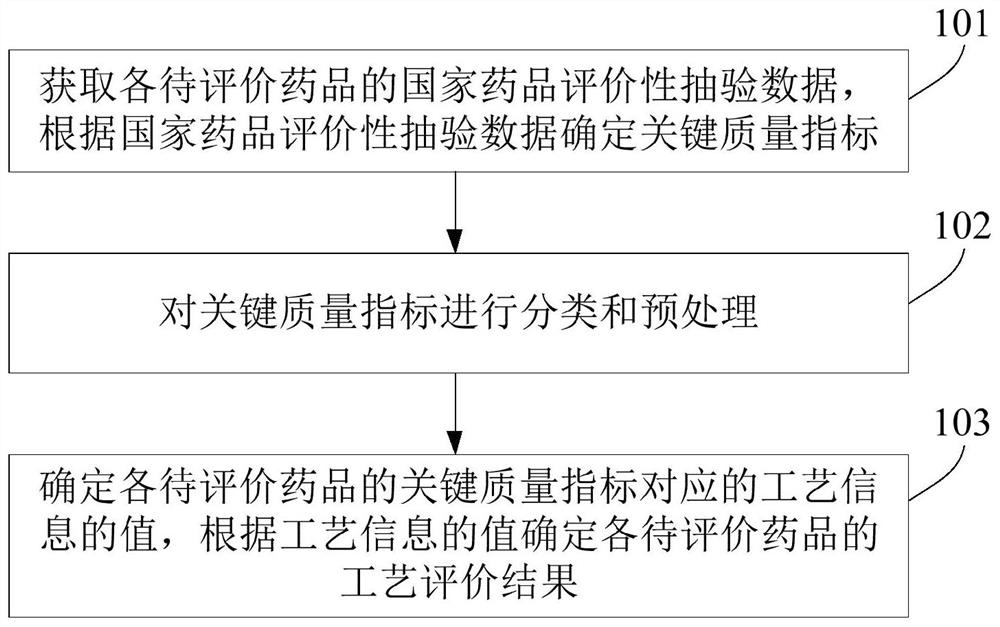
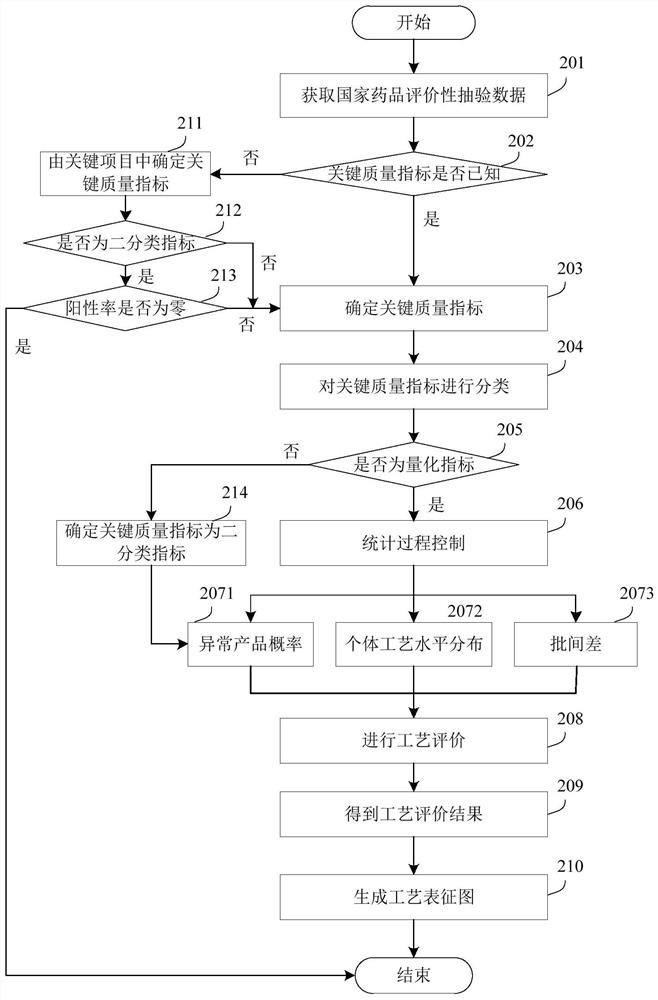
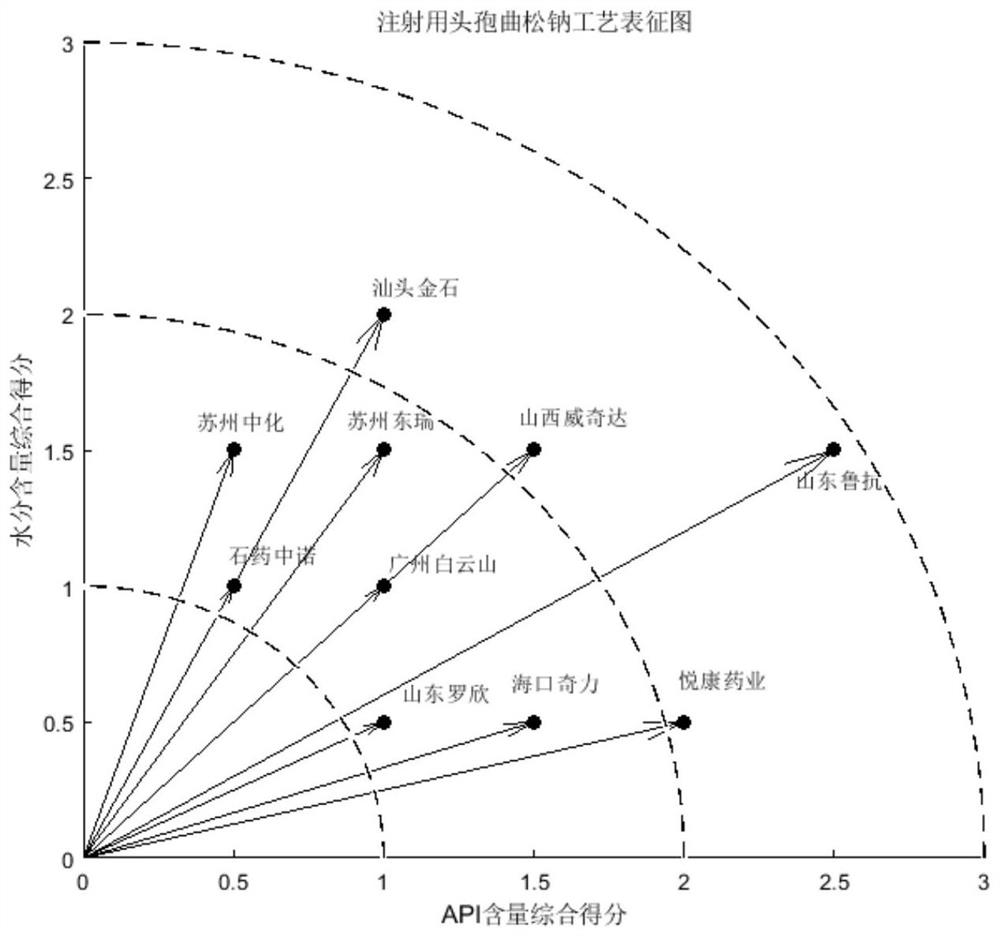
Process evaluation method and system for imitating drugs
The embodiment of the invention relates to a process evaluation method and system for imitating a medicine, and aims to realize more comprehensive process evaluation on the medicine. Furthermore, theprocess information of the key quality index can be better represented. The method comprises the following steps: acquiring national drug evaluation sampling data of each to-be-evaluated drug, and determining a key quality index according to the national drug evaluation sampling data; classifying and preprocessing the key quality indexes; determining a value of process information corresponding tothe key quality index of each to-be-evaluated drug, and determining a process evaluation result of each to-be-evaluated drug according to the value of the process information, wherein the process information comprises individual process level distribution, inter-batch difference and abnormal product probability.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Establishment method and application of novel immunological hepatitis model
InactiveCN103920165AHigh simulationShort experiment cycleIn-vivo testing preparationsAnimal husbandryAntigenHepatitis B Virus Antigen
The invention relates to preparation method for an immunological hepatitis animal model induced by hepatitis B virus whole genome transfected mouse hepatocytes, especially to an immunological inducer containing hepatocyte autoantigens and hepatitis B virus antigens and a simple operation procedure, generation of an immunological hepatitis disease model more similar to human diseases and application of the model in hepatitis pathogenesis study and anti-hepatitis drug evaluation.
Owner:CHINA JILIANG UNIV
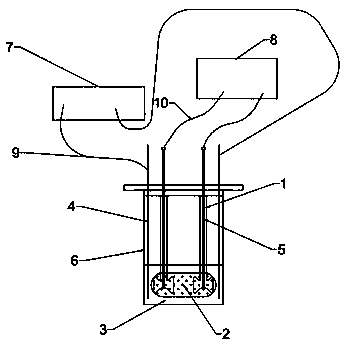
Optical fiber measurement based three-dimensional heart chip and detection method thereof
ActiveCN110231468AResponse true reflectionMyocardial beating frequency sensitiveConverting sensor output opticallyMaterial analysisMedicineCardiac muscle
The invention discloses an optical fiber measurement based three-dimensional heart chip. According to the chip, elastic cantilevers serve as a support for three-dimensional myocardial tissue and can be continuously deformed and displaced in the contraction and relaxation processes of the three-dimensional myocardial tissue so as to be detected by optic fiber probes in the elastic cantilevers; under the action of contraction and relaxation behaviors of the three-dimensional myocardial tissue, the two cantilevers repeatedly get close and move far away, the distance between the two cantilevers ischanged and the characteristics of the beating frequency, the contraction force and the like of the myocardial tissue are reflected by the change frequency and the displacement amplitude; the changeof the elastic cantilevers in which optical fiber is wrapped is monitored by utilizing the characteristics of sensitivity, accuracy and the like of optical fiber measurement technology, thereby realizing the detection of important indexes such as the myocardial beating frequency, the contraction force and the like; and after a drug is added, the indexes such as the beating frequency, the contraction force and the like of the three-dimensional myocardial tissue are changed, drug evaluation and screening can be carried out by utilizing the data obtained by the optical fiber monitoring, the effect of the drug on myocardium can be observed in real time and a good visualization effect is provided.
Owner:INST OF MEDICAL DEVICES (SUZHOU) SOUTHEAST UNIV
Construction method of drug evaluation model for dermal pathology of tuberculosis rabbit
InactiveCN102430119AObvious symptomsOrganic active ingredientsBacterial antigen ingredientsBCG immunizationTreating tuberculosis
The invention discloses a construction method of a drug evaluation model for dermal pathology of a tuberculosis rabbit. The method comprises: selecting a rabbit, injecting an immune drug into rabbit, conducting BCG (Bacillus Calmette Guerin) vaccine immune injection on the 15th to 20th day of drug intervention, and carrying out bacterial and pathological examination so as to construct a pathological model. The method of the invention establishes a pathological model of drug intervention on drug cutaneous tuberculosis with obvious symptoms so as to conduct visual research on tuberculosis pathology and bacterial pathogenicity, thus providing a visual animal model for vaccines and screening of drugs treating a tuberculosis necrotic liquefied cavity. And the model is stable and repeatable. The invention establishes a drug intervention procedure and observation indexes for research of drug intervention on a tuberculosis granuloma liquefaction process, and also provides a research basis for probing an immune mechanism about the formation of a tuberculosis liquefied cavity.
Owner:LANZHOU UNIVERSITY
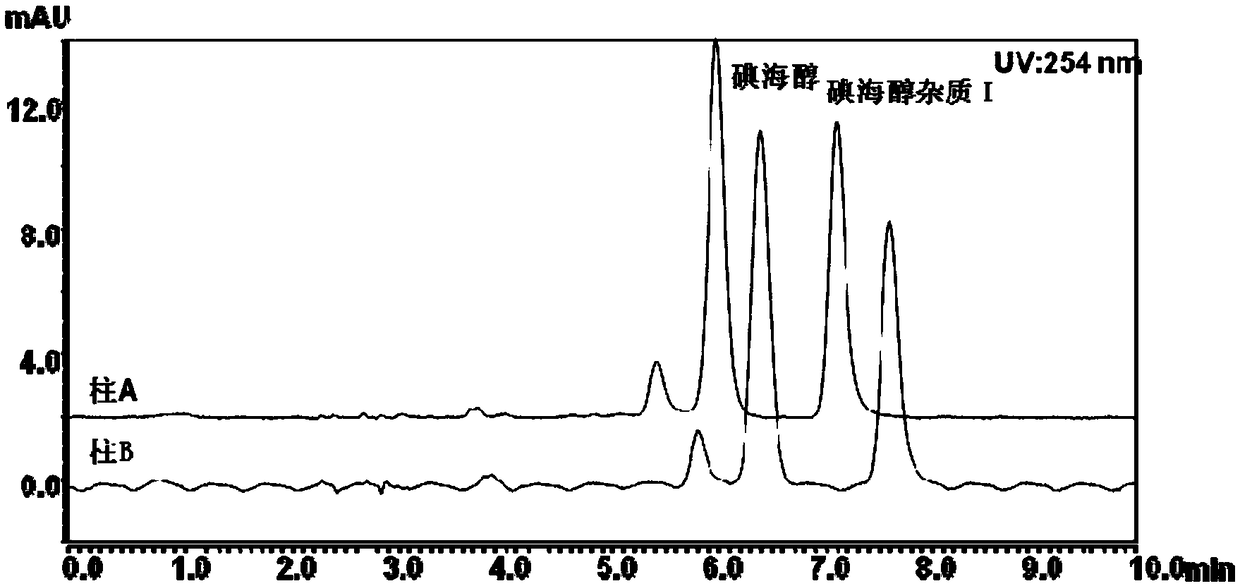
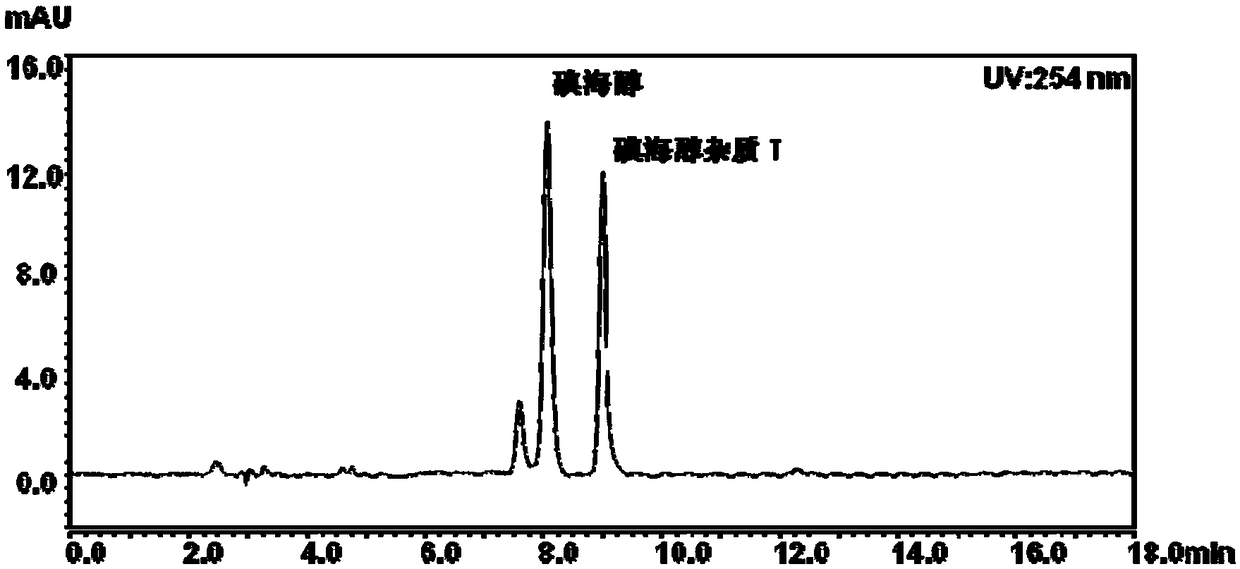
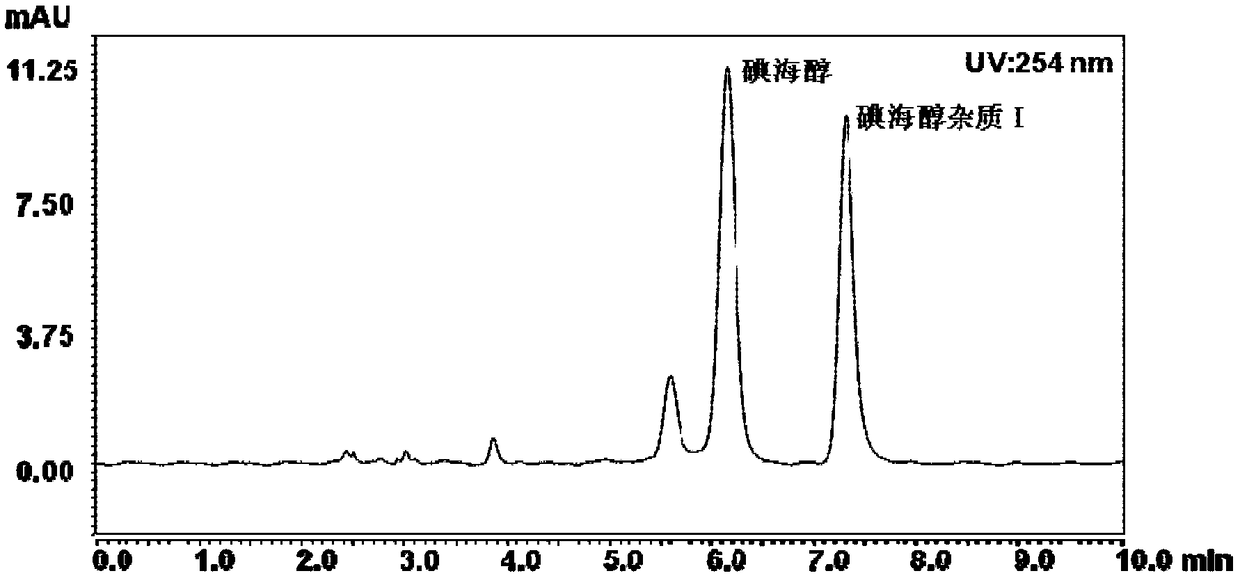
Method for detecting content of iohexol in plasma of rhesus monkeys and application thereof to evaluation of influence of drug on GFR (Glomerular Filtration Rate)
The invention discloses an exclusive method for detecting the content of iohexol in plasma of rhesus monkeys and application thereof to evaluation of the influence of a drug on GFR (Glomerular Filtration Rate), and belongs to the field of drug evaluation. The exclusive method comprises the following steps: (1) standard product working solution and standard curve preparation: preparing standard production working solutions in various concentrations; (2) preparation of an internal standard production solution preparing an iohexol impurity I working solution; (3) sample pretreatment; (4) determination of iodexol: determining under the conditions that a C18 is used as a chromatography column, a mobile phase comprises a mobile phase A which is methanol and a mobile phase B which is phosphoric acid water, isocratic elution with elution time of 10 minutes , 20 to 40 DEG C in temperature and 0.2 to 1.5 mL / min in flowing speed, the detection wavelength of a chromatogram is 190 to 320 nm and thesampling volume is 1 to 20 mu L. The exclusive method disclosed by the invention is capable of detecting the content of the iohexol, is accurate and simple and is time-saving and labor-saving; the dosage of the iohexol required for determining and evaluating the GFR by utilizing the iodexol clearance rate is smaller, and the safety of kidney is greatly improved; the exclusive method is simple andreliable, the exclusiveness is strong, and effective experimental data can be provided for clinical application.
Owner:SICHUAN PRIMED BIO TECH CO LTD
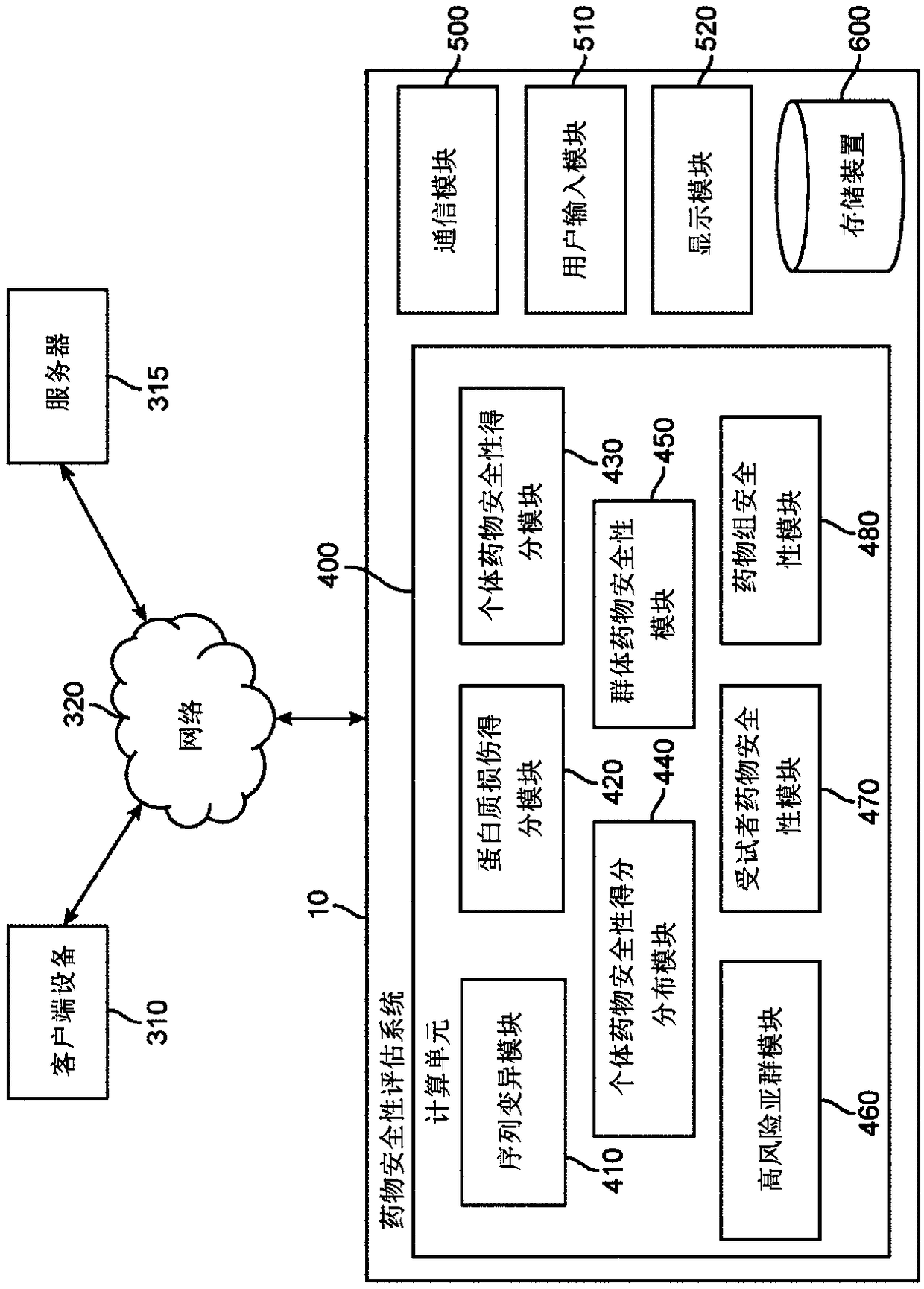
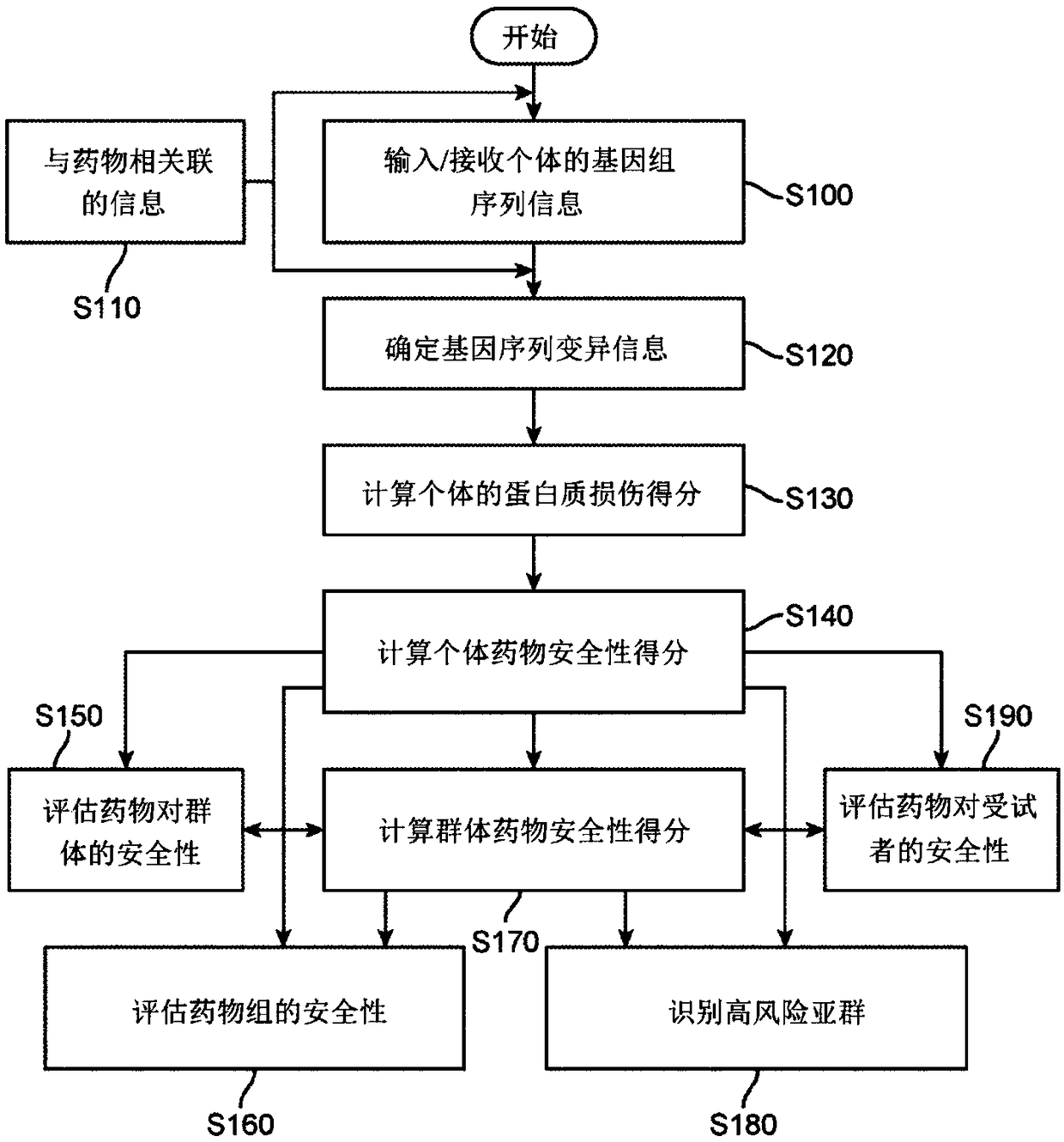
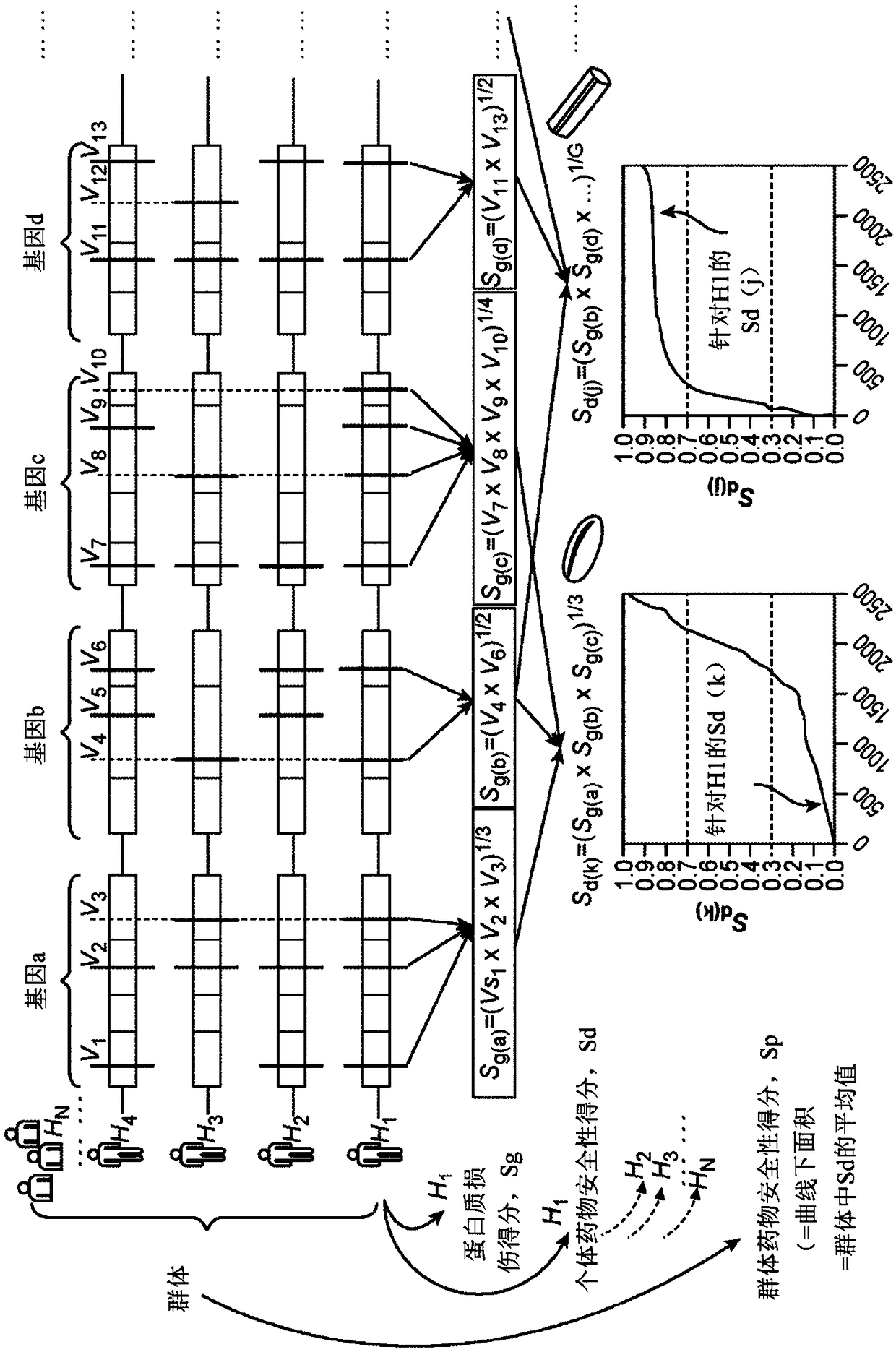
Computer-implemented evaluaton of drug safety for a population
A computer-implemented drug evaluation method and system provides for evaluating safety of a drug or a drug group by performing certain computations associated with gene sequence variation informationof individuals within a population. The system calculates various scores for individual within a population and ultimately combines the scores in determining safety of the drug across the population.The drug evaluation method and a system can further be configured for identifying individuals having a high-risk of side effects to a drug or a drug group. The drug evaluation provides universal drugsafety information based on gene sequence variation information without the need to identify specific genetic markers for each drug.
Owner:CIPHEROME INC
Methods for multiplexed drug evaluation
InactiveUS20140170146A1Compound screeningApoptosis detectionSolid tissueMass Spectrometry-Mass Spectrometry
Owner:PRESAGE BIOSCI
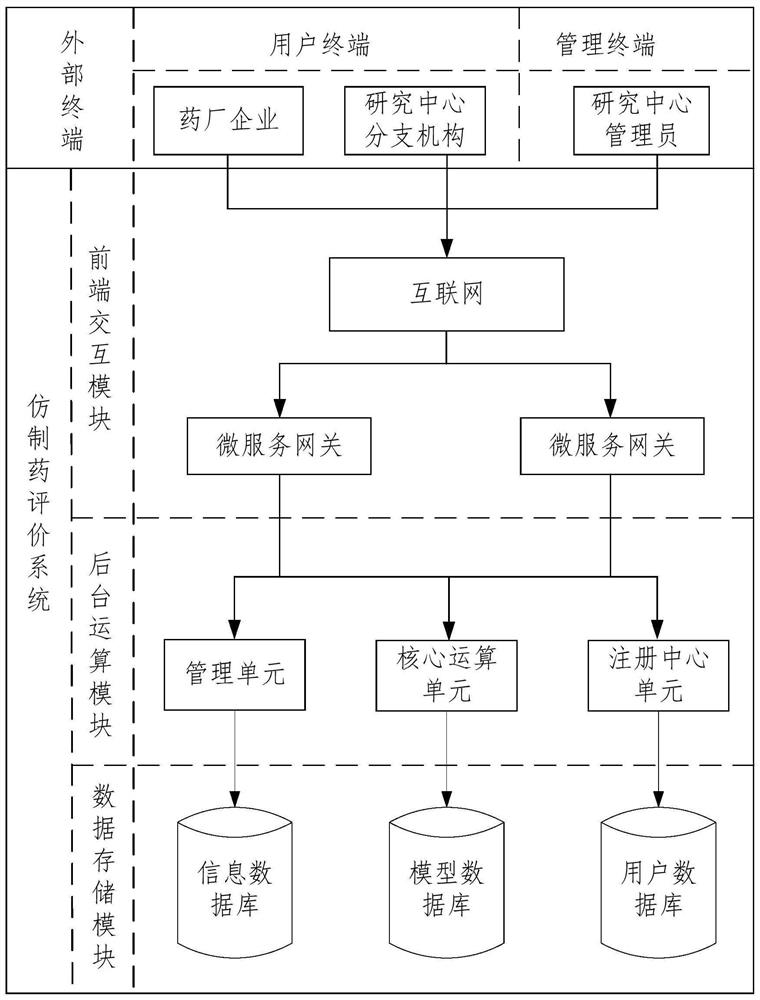
Drug evaluation system and method applied to cloud platform
PendingCN113705942AImprove objectivityImprove accuracyDatabase management systemsResourcesEvaluation resultMedicine
The invention provides a drug evaluation system and method applied to a cloud platform, and relates to the technical field of medicines.The system obtains drug original information sent by an external terminal and evaluation request information of a to-be-evaluated drug through a front-end interaction module, and sends the drug original information and the evaluation request information to the background operation module through the micro-service gateway; a background operation module is used for evaluating the to-be-evaluated medicine according to the evaluation request information and the medicine original information to obtain medicine evaluation information of the to-be-evaluated medicine, and sending the medicine original information and the medicine evaluation information to a data storage module for storage. According to the method and the device, more comprehensive drug original information can be obtained, and the objectivity and the accuracy of an evaluation result are effectively improved.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Method for evaluating preventing effect of drug on viral pneumonia
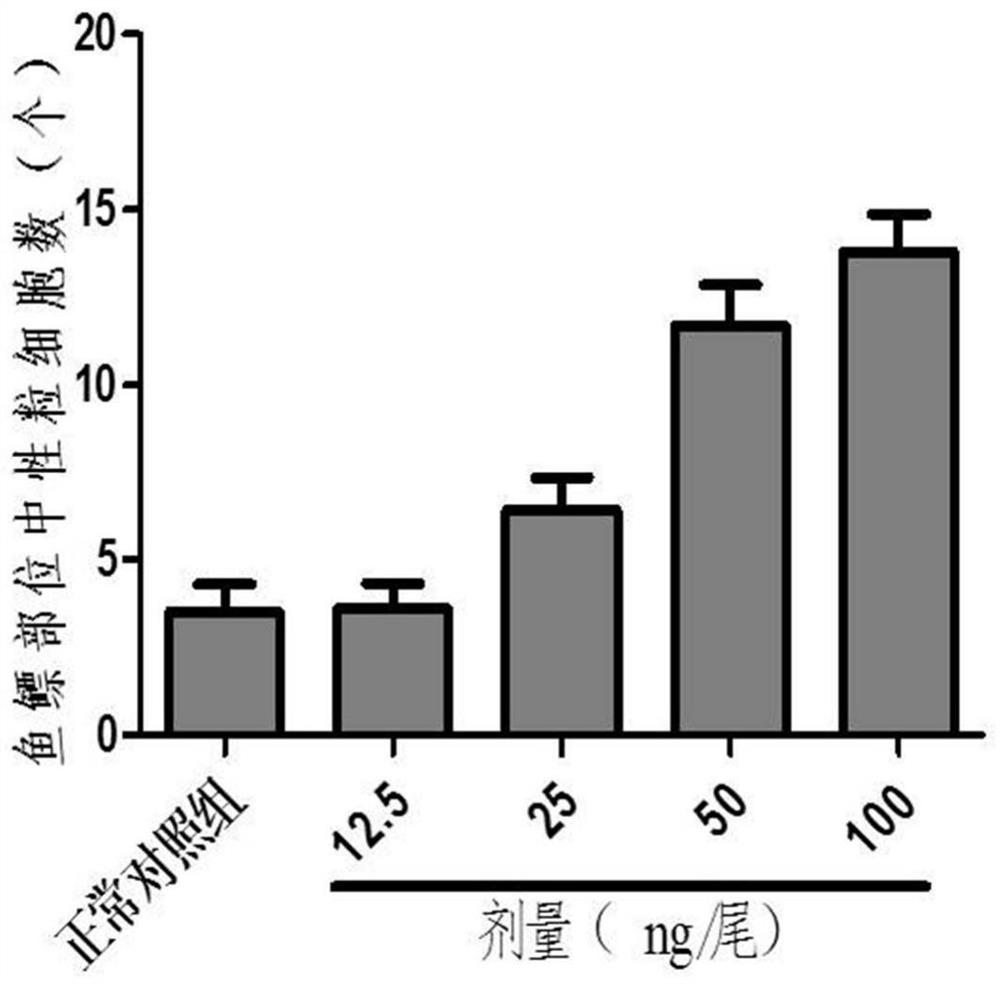
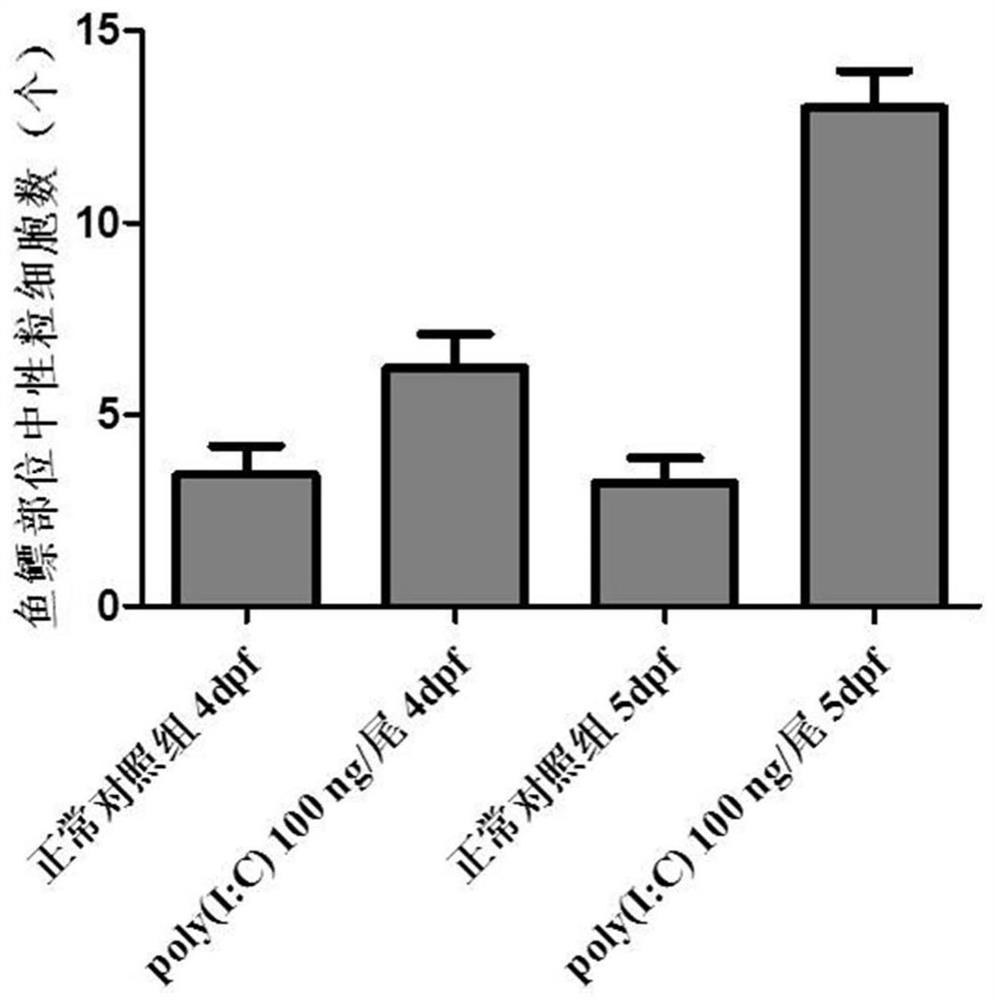
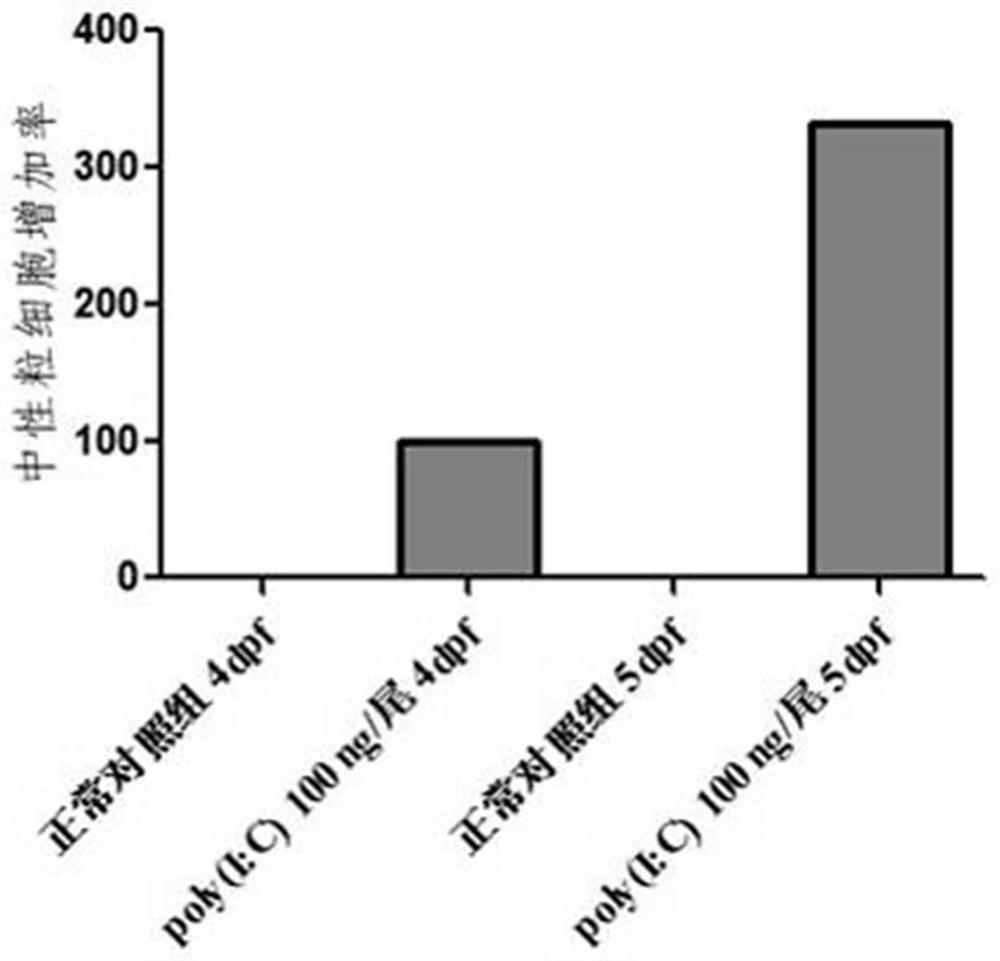
ActiveCN112106705AShort development cycleSmall number of eggs per spawnCompounds screening/testingClimate change adaptationDrugs evaluationsRNA
The invention relates to the technical field of drug evaluation and screening, discloses a method for establishing a pneumonia animal model, and provides a method for evaluating a preventing effect ofa drug on viral pneumonia by reasonably adopting the model to solve the problems that an existing pneumonia evaluation animal model is complex in modeling operation and not beneficial to subsequent evaluation and screening work of a pneumonia treatment drug. The pneumonia animal model is established by placing zebra fishes of 5dpf in water for culturing and injecting a pneumonia inducer poly(I:C)into the zebra fishes, wherein the administration concentration is greater than or equal to 50 ng per zebra fish; and indexes are evaluated by adopting the inflammatory regression effect, the macrophage improvement effect and the related gene RNA relative expression quantity effect during drug evaluation. The pneumonia model can be used for evaluating the toxicity or efficacy of known drugs can be further used for screening unknown drugs, the real situation of the drugs in vivo can be accurately reflected, in-vivo high-throughput screening or evaluating of the treatment effect of the drugs onviral pneumonia can be realized, and the advantages of reliability, rapidness, high efficiency, low cost, high cost performance and the like are achieved.
Owner:北京环特智鱼优检生物科技有限公司
DNA (Deoxyribonucleic acid) segment and applications thereof in constructing HCV (hepatitis C virus) whole-genome expression animal model
The invention discloses a DNA (deoxyribonucleic acid) segment and applications thereof in constructing an HCV (hepatitis C virus) whole-genome expression animal model. The nucleotide sequence of the DNA segment comprises the following sequentially series connected sequences: an operator gene sequence of bacteria tetracycline drug-resistance operon, an immediate early promoter gene sequence of human macrophage, a gene sequence of HCV whole genome, and a gene sequence of ribozyme shearing the HCV whole genome activity on DNA and / or RNA (ribonucleic acid) level. The DNA segment can be used for constructing a tetracycline or tetracycline derivative-induced HCV whole genome-expressed transgenic animal model. The immunity of the animal model is normal, thereby being capable of producing specific anti-virus immunoreaction aiming at the expression of the HCV, and further being effectively applied to the research on the immunopathogenesis damage mechanism of drug evaluation and virus infection of the HCV vaccine.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Micro-fluid driving method applied to organ chip
InactiveCN107955788AIncrease fluid residence timeIncrease contact areaBioreactor/fermenter combinationsBiological substance pretreatmentsFluidicsEngineering
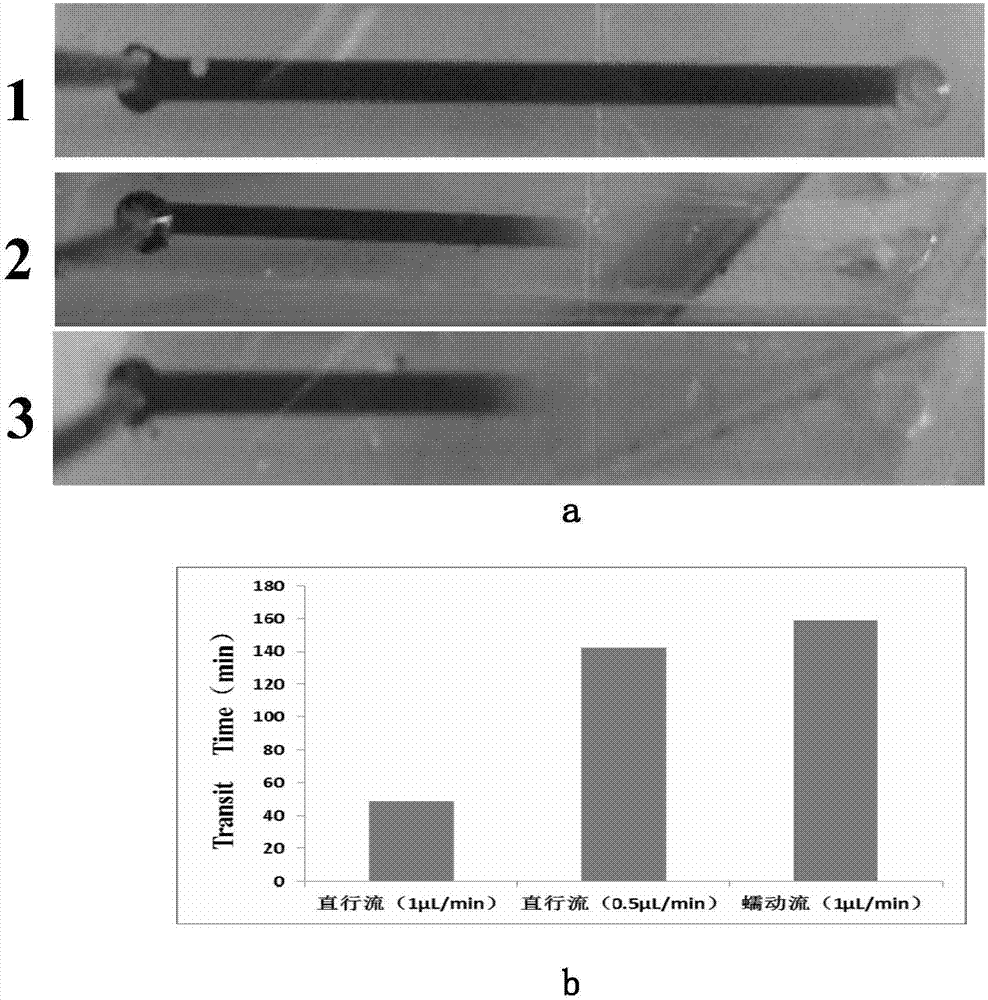
The invention provides a micro-fluid driving method applied to an organ chip, and belongs to the technical field of micro-fluidics. According to the method, motion modes, namely alternated moving forwards and backwards, of fluid on the organ chip are controlled by virtue of a control pump; a peristaltic flow is formed in a micro-channel of the organ chip; and through the peristaltic flow, bidirectional shear force of the fluid is presented, the retention duration of the fluid in the micro-channel is prolonged and a fluid contact area is increased, so that a better fluid mixing effect is achieved. The driving method is stable in effect; fluid, in a peristaltic mode, in an organ can be simulated, so that micro-fluid functions are enriched; construction of the organ chip and a micro-reactor in an in-vitro simulation environment can be achieved; and the method has an important significance for application to drug evaluation and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of standardized in vitro tumor micro-tissues
InactiveCN107421791ASolving problems that cannot be standardizedEasy to comparePreparing sample for investigationIn vitro digestionMedicine
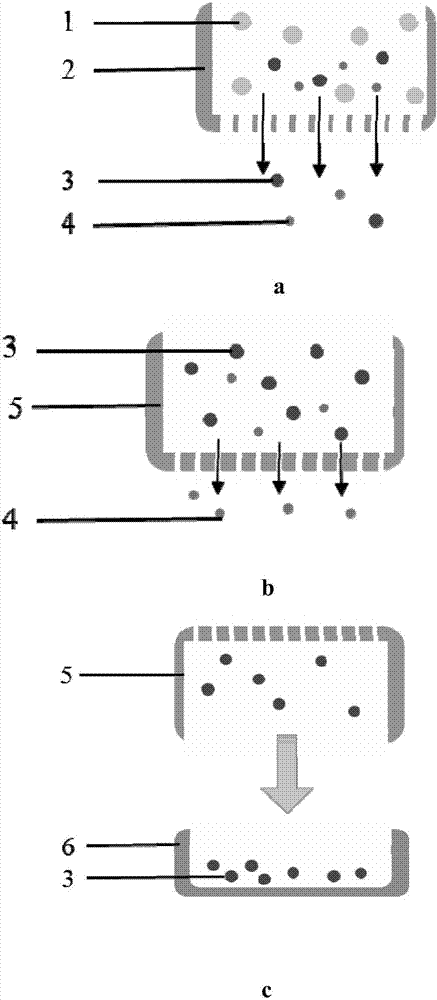
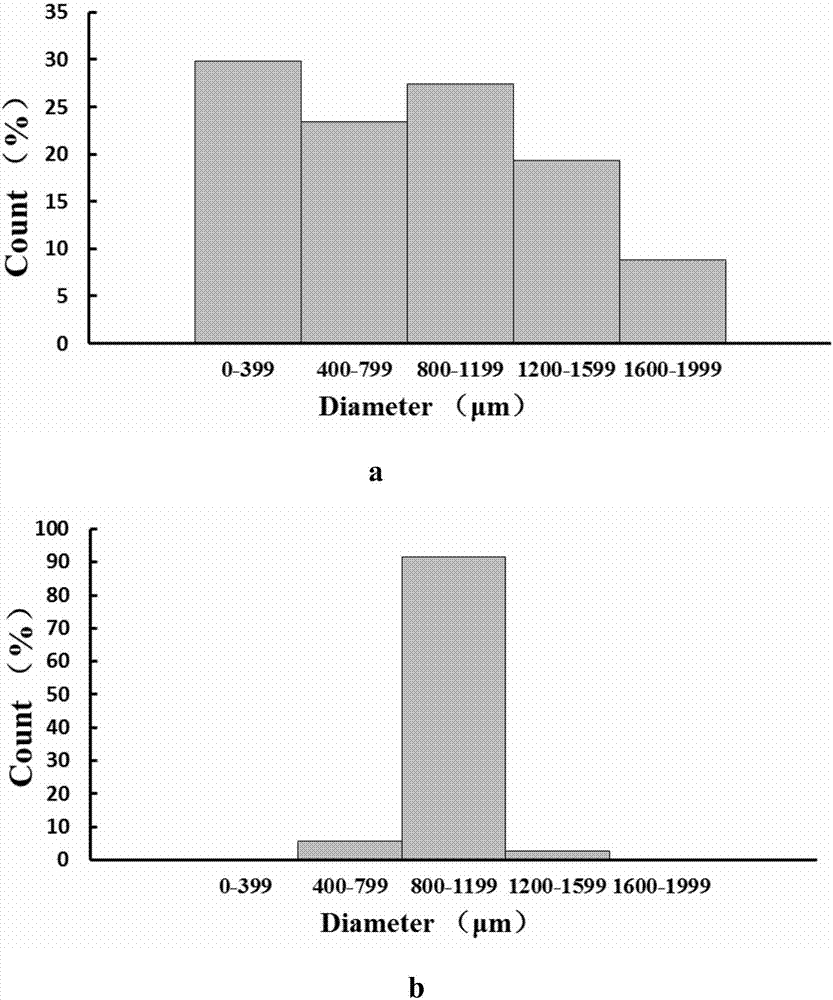
The invention provides a preparation method of standardized in vitro tumor micro-tissues, belonging to the technical field of biological medicines. The preparation method comprises the steps of dividing a solid tumor tissue into small blocks, and carrying out interception by virtue of a porous sieve according to the diameter size of the tumors, so as to obtain uniform-size tumor micro-tissues. The preparation method can be applied to the culture and drug evaluation of tumor tissues. The tumor tissues prepared by virtue of the preparation method are controllable in sizes and convenient to use and can be standardized for subsequent operation. According to the preparation method, the problem that the in vitro culture of the tumor tissues is difficult to be standardized is solved; and meanwhile, the used tumor tissues are not subjected to in vitro digestion and dispersion, so that the components and characters of the tumor tissues are preserved, and the effects of the tumor tissues are guaranteed.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A process evaluation method and system for generic drugs
The embodiment of the present invention relates to a process evaluation method and system for generic drugs, so as to realize a more comprehensive process evaluation of drugs; further, it can better characterize the process information of key quality indicators. The method includes: obtaining national drug evaluation random test data of each drug to be evaluated, determining key quality indicators according to the national drug evaluation random test data; classifying and preprocessing the key quality indicators; determining each drug to be evaluated According to the value of the process information corresponding to the key quality index, the process evaluation result of each drug to be evaluated is determined according to the value of the process information; the process information includes individual process level distribution, inter-batch difference and abnormal product probability.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Therapeutic methods and compositions for solid delivery
An administration device comprising an array of needles, one or more fluid agents, and at least one hydrogel is described. The device can simultaneously deliver a plurality of fluid agents along respective axes into a tissue. The use of hydrogel leads to constrained delivery of the fluid agents. The constrained delivery of an agent is also achieved by depositing a drug implant into a tissue. The effect of an agent on the tissue can be evaluated thereafter. In addition, the invention is directed to treating muscle diseases by delivering a therapeutic agent in vivo, and the use of reporter tissues for candidate drug evaluation, detecting and characterizing resistance.
Owner:PRESAGE BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com