Method for establishing humanized rat drug evaluation animal model
A human and construct technology, applied in the direction of using vectors to introduce foreign genetic material, recombinant DNA technology, animal husbandry, etc., can solve problems such as differences in expression profiles, differences in gene regulation, and limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Production of Transgenic Rats Expressing Abcb1 Bacterial Artificial Chromosomes
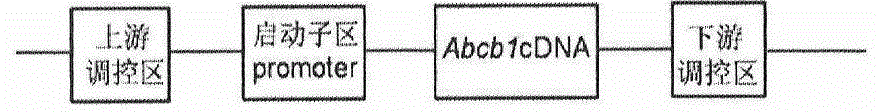
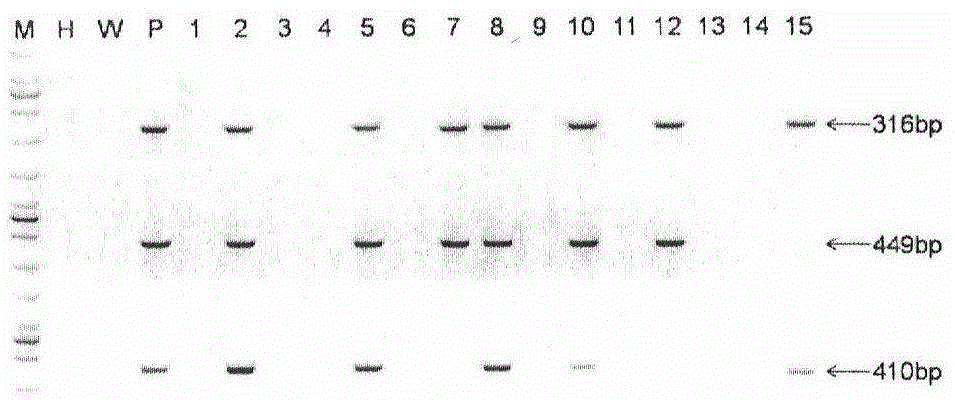
[0024] The longest spliced cDNA (4718bp) of the human Abcb1 gene and its upstream and downstream regulatory sequences (upstream length is 66kbp, ATG downstream length is 87kbp) were cloned into the bacterial artificial chromosome BAC, and the BAC expression vector expressing Abcb1 was completed. After the constructed carrier was extracted by phenol-chloroform method, the concentration was adjusted to 1-2ng / μl, BAC was injected into fertilized eggs of SD rats by microinjection technique, and SD rats were used as pseudopregnant recipient rats , to prepare transgenic rats. A total of 15 F0 generation rats were obtained, and the genomic DNA of the born rats was extracted. In order to detect its integrity, three pairs of PCR primers were used to detect the integrity of the upper, middle and lower reaches of the inserted BAC, respectively: Abcb1-BAC-F1: 5'-CACCAGTAAGAGCGTTGA-3' Dow...
Embodiment 2
[0025] Example 2: Production of Abcb1 knockout rats
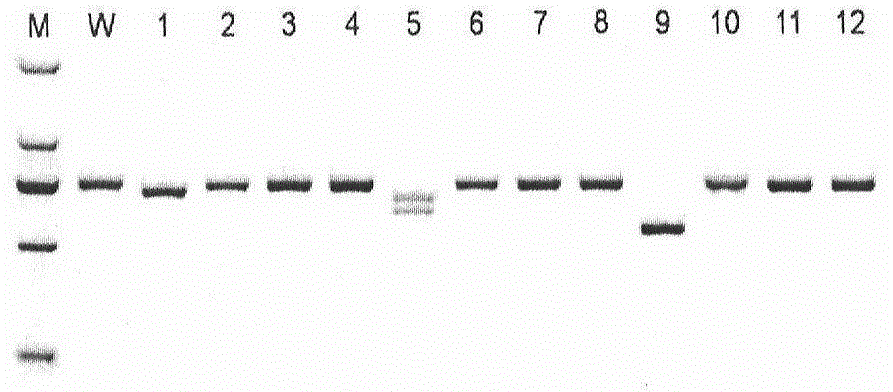
[0026] Construction of gRNA plasmid for Abcb1 gene targeting: a target GGAGACAAATACACAAGATT was designed for the Abcb1 gene, and a pair of oligonucleotide chains (TAGGAGACAAATACACAAGATT and AAACAATCTTGTGTATTTGTCT; used to prepare sgRNA were synthesized; the synthesized oligonucleotide was annealed (97 After 6 mins at ℃ and naturally lowered to room temperature), connect into the pUC57-sgRNA expression vector recovered by Bsa I enzyme digestion to construct the sgRNA expression vector. Verify whether the connected fragment is correct by sequencing, select the correct clone, and prepare for use after expanding the culture Later in vitro transcription.
[0027] In vitro transcription: the Cas9 expression plasmid was digested with Age I and linearized, extracted and purified with phenol chloroform, dissolved in nuclease-free water as a template, and used for in vitro transcription. The synthesis of Cas9 mRNA was completed by T...
Embodiment 3
[0029] Embodiment 3: the cultivation of humanized Abcb1 rat
[0030] rAbcb1 gene knockout homozygous rats can be obtained by crossing the obtained rAbcb1 gene knockout rats, and BAC positive and rAbcb1 gene knockout rats can be obtained by crossing hAbcb1-BAC positive rats with rAbcb1 gene knockout rats In addition to the heterozygous rats, by crossing this strain of rats with rAbcb1 gene knockout homozygous rats, hAbcb1-BAC positive and rAbcb1 gene knockout homozygous rats can be obtained. This kind of rat lacks the expression of its own Abcb1 gene at the gene level, and at the same time, it also carries the human Abcbl gene obtained through BAC technology.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com