Method for preparing aldehyde/ketone by catalyzing alcohol oxidation with ferric salt
A technology for ferric salts to catalyze alcohols and ferric salts. It is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of limited substrate range and inapplicability of acid-sensitive substrates. broad effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
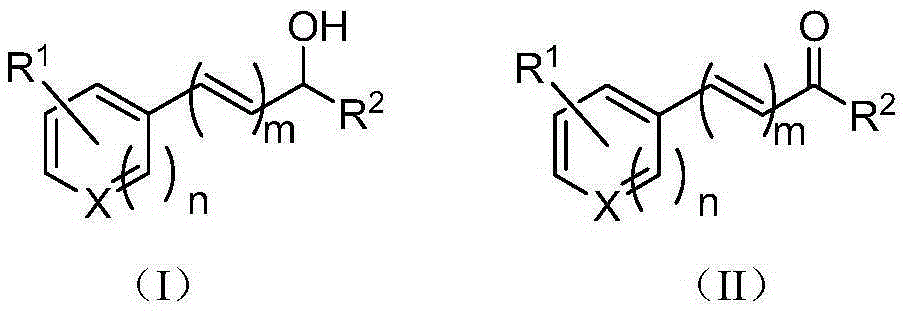
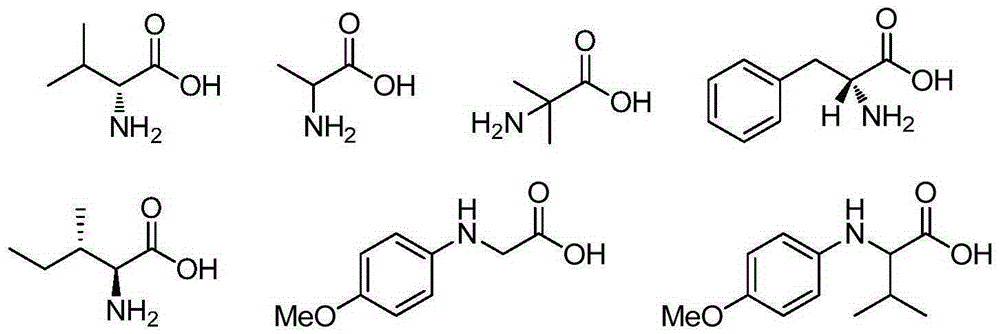
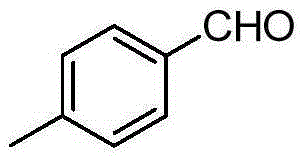
[0038] Add p-methylbenzyl alcohol (12.22g, 100.0mmol, R in the formula (I) 1 is 4-methyl, R 2 hydrogen, X is carbon, n is 1, m is 0), ferric chloride (0.81g, 5mmol), L-isoleucine (1.31g, 10mmol), TEMPO (1.56g, 10mmol), toluene 300.0mL , and then replace the air in the reaction flask with oxygen, and stir at reflux for 6h. After the reaction, the reaction solution was cooled to room temperature, filtered, and the filtrate was evaporated to remove the solvent to obtain a crude product, which was purified by column chromatography and eluted with a mixture of n-hexane:ethyl acetate (volume ratio 10:1) , collected the eluate containing the target compound, evaporated the solvent and dried to obtain 10.93 g of the product p-tolualdehyde with a yield of 91%.
[0039] H NMR spectrum 1 HNMR (500MHz, CDCl 3 ): δ2.38(s,3H),7.27(d,J=4.3Hz,2H),7.73(d,J=4.0Hz,2H),9.91(s,1H).
[0040] C NMR spectrum 13 CNMR (125MHz, CDCl 3 ): δ21.5, 129.4, 129.5, 134.0, 145.2, 191.6.
Embodiment 2
[0042]
[0043] The reactant used is p-methoxybenzyl alcohol (12.22g, 100.0mmol, R in the formula (I) 1 is 4-methoxy, R 2 Be hydrogen, X is carbon, n is 1, m is 0), experimental method and step are with embodiment 1, difference is: ferrous chloride (2.54g, 20mmol), L-isoleucine (1.31g , 10mmol), TEMPO (1.56g, 10mmol), chlorobenzene 300.0mL, reflux and stir under air condition for 24h. Finally, 12.53 g of the product was obtained with a yield of 92%.
[0044] H NMR spectrum 1 HNMR (500MHz, CDCl 3 ): δ3.71(s,3H),6.84(d,J=4.3Hz,2H),7.67(d,J=4.3Hz,2H),9.72(s,1H).
[0045] C NMR spectrum 13 CNMR (125MHz, CDCl 3 ): δ55.4, 114.2, 129.9, 131.8, 164.6, 190.6.
Embodiment 3
[0047]
[0048] The reactant used is 3,4,5-trimethoxybenzyl alcohol (12.22g, 100.0mmol, R in formula (I) 1 is 3,4,5-trimethoxy, R 2 Be hydrogen, X is carbon, n is 1, m is 0), experimental method and step are with embodiment 1, difference is: iron trifluoromethanesulfonate (25.15g, 50mmol), 2-methylalanine (5.16g, 50mmol), TEMPO (4.69g, 30mmol), xylene 300.0mL, replace the air in the reaction bottle with oxygen, and then reflux and stir for 1h. Finally, 18.05 g of the product was obtained with a yield of 92%.
[0049] H NMR spectrum 1 HNMR (500MHz, CDCl 3 ): δ3.89(d,J=4.0Hz,9H),7.09(s,2H),9.83(s,1H).
[0050] C NMR spectrum 13 CNMR (125MHz, CDCl 3 ): δ56.2, 60.8, 106.7, 131.7, 143.6, 153.6, 190.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com