A kind of preparation method of aralkyl salicylic acid derivative
A technology of aralkylsalicylic acid and salicylic acid derivatives, which is applied in the field of preparation of aralkylsalicylic acid derivatives, can solve the problems of environmental pollution, easy residual catalyst, slow color development speed and the like, and achieves green synthesis process, environmental protection, and the effect of convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
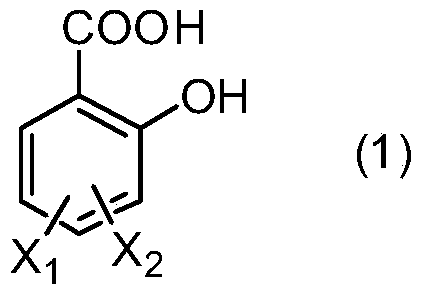
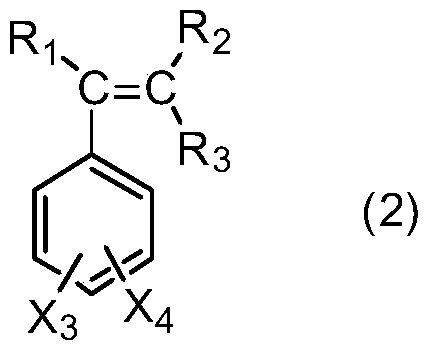
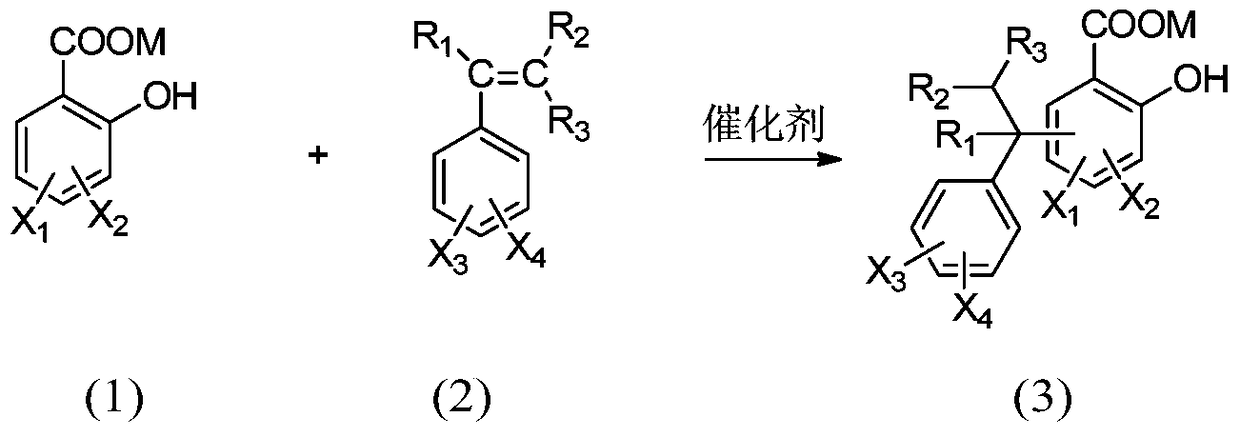
[0038] In a 500ml three-necked flask equipped with a condenser, a thermometer and a mechanical stirrer, add 1-(3-sulfonic acid) propyl-3-methylimidazolium bisulfate ([C 3 SO 3 Hmim][HSO 4]) ionic liquid 10g, salicylic acid derivative 138.1g (1.0mol) as shown in formula (1), take by weighing 229.2g (2.2mol) of styrene derivative as shown in formula (2), first drop into 20g of styrene derivatives, heat up to 120°C, stir rapidly, then add the remaining part of styrene derivatives dropwise, drop for 8 hours, and react for 1 hour after dropping, stop stirring, static layering, phase separation to remove the lower catalyst, the upper layer The viscous material was distilled off under reduced pressure to remove unreacted olefins to obtain yellow viscous liquid aralkyl salicylic acid with a yield of 96.5%.
[0039] Among the above reaction materials,
[0040] M, X in formula (1) 1 and x 2 Both are H;
[0041] R in formula (2) 1 , R 2 , R 3 、X 3 、X 4 Both are H.
Embodiment 2
[0043] In a 500ml three-necked flask equipped with a condenser, a thermometer and a mechanical stirrer, add 1-(4-sulfonic acid) butyl-3-methylimidazolium bisulfate ([C 4 SO 3 Hmim][HSO 4 ]) ionic liquid 10g, salicylic acid derivative 166.2g (1.0mol) as shown in formula (1), take by weighing 130.0g (1.1mol) of styrene derivative as shown in formula (2), first drop into 20g of styrene derivatives, heat up to 100°C, stir rapidly, then add the remaining part of styrene derivatives dropwise, drop for 6 hours, react for 0.5 hours after dropping, stop stirring, static layering, phase separation to remove the lower catalyst, the upper layer The viscous material was distilled off under reduced pressure to remove unreacted olefins to obtain a yellow viscous liquid aralkyl salicylic acid derivative with a yield of 97.1%.
[0044] Among the above reaction materials,
[0045] In formula (1), M is methyl, X 1 for H, X 2 is 4-methyl;
[0046] R in formula (2) 1 is methyl, R 2 , R 3 ...
Embodiment 3
[0048] In a 500ml three-necked flask equipped with a condenser, a thermometer and a mechanical stirrer, add 1,1,3,3-tetramethyl-2-propanesulfonate guanidine bisulfate ([C 3 SO 3 TMGH][HSO 4 ]) ionic liquid 10g, salicylic acid derivative 182.2g (1.0mol) as shown in formula (1), take by weighing 176.3g (1.1mol) of styrene derivative as shown in formula (2), first drop into 20g of styrene derivatives, heat up to 140°C, stir rapidly, then add the remaining part of styrene derivatives dropwise, drop for 10 hours, react for 2 hours after dropping, stop stirring, static layering, phase separation to remove the lower catalyst, the upper layer The viscous material was distilled off under reduced pressure to remove unreacted olefins to obtain yellow viscous liquid aralkyl salicylic acid with a yield of 96.1%.
[0049] Among the above reaction materials,
[0050] In formula (1), M is n-butyl, X 1 is H, X 2 is 4-methoxy;
[0051] R in formula (2) 1 , R 2 , R 3 、X 3 is H, X 4 Fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com