Hcy structural analog aht that inhibits methionyl-tRNA synthetase to produce htl and its application
A technology with a similar structure and methionyl, applied in the biological field, can solve problems such as increased reactive oxygen species and increased Hcy levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of AHT:
[0037]
[0038] Weigh 5 g DL-homocysteine into a 250 mL round bottom flask, N 2 For replacement, add 160 mL of anhydrous methanol, and add SOCl dropwise under ice-cooling 2 4 mL, and stirred for 24 h at room temperature with the ice removed.
[0039] After stopping the reaction, the solvent was spin-dried. The crude product was dissolved in DCM, and 10.35 mL of Et 3 N, then join Boc 2 O 8.9 g, stirred at room temperature for 2.5 h.
[0040] After stopping the reaction, the solvent was spin-dried. The crude product plus CH 3 80 mL of OH, H 2 O 35 mL, add 9.2 mL of tributylphosphine dropwise, and stir at 50°C for 30 minutes. The reaction system with 200 mL ether, 100 mL H 2 O was diluted and the organic phase was separated. The aqueous phase was extracted twice with ether (2×100 mL), the organic phases were combined, washed with saturated brine, anhydrous Na 2 SO 4 After drying and spin-drying, a colorless oil (compo...
Embodiment 2
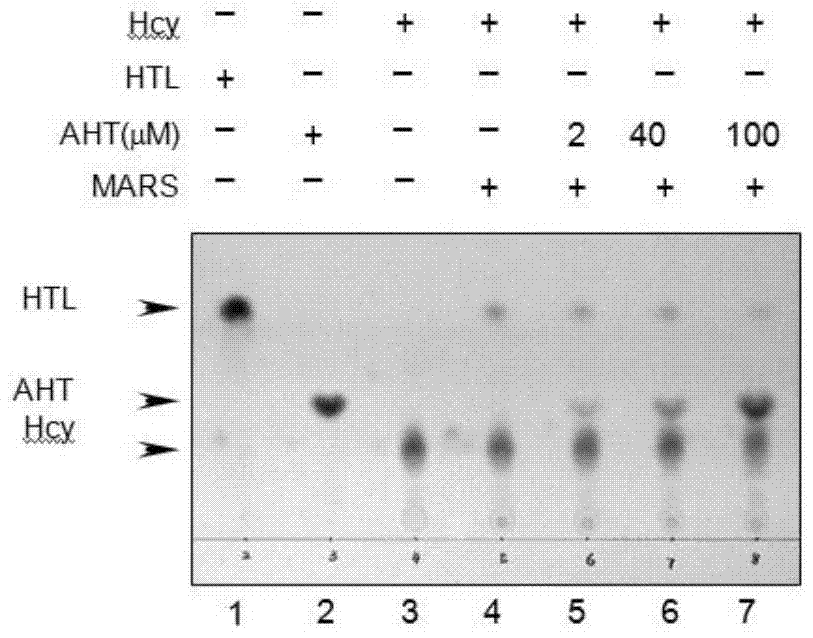
[0047] Example 2. Thin-layer chromatography experiments verify that AHT, a structural analogue of Hcy, can inhibit MARS from converting Hcy into HTL in vitro.
[0048] Reaction system configuration:
[0049] 50mM Hepes (pH8.0), 10mM MgCl 2 ,10mM 2-mercaptoethanol, 1.5mM ATP, 5mM homocysteine, 30μM methionyl tRNA synthetase, AHT, react at 25 for 100min
[0050] Thin Layer Chromatography Detection of HTL Formation
[0051] Spotting: Use a pencil to draw a straight line 2cm away from one end of the silica gel plate and make a spotting mark. Use a micropipette to draw 4 ul sample point to the marked sample addition point, the diameter of each point shall not be greater than 0.5cm, and dry it with cold air.
[0052] Development: prepare the developing agent according to n-butanol: glacial acetic acid: water = 4:1:1, mix evenly and pour it into the developing tank, place the spotted thin plate in the developing reagent color developing solution in the chromatography tank, and sea...
Embodiment 3
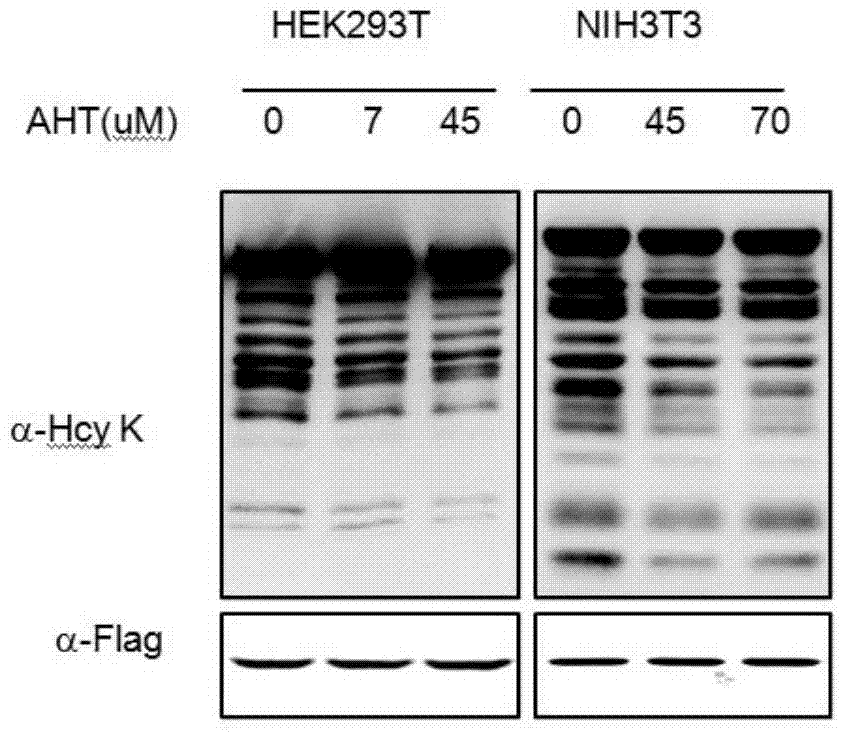
[0055] Example 3, the influence of AHT on the homocysteine modification of total cell protein
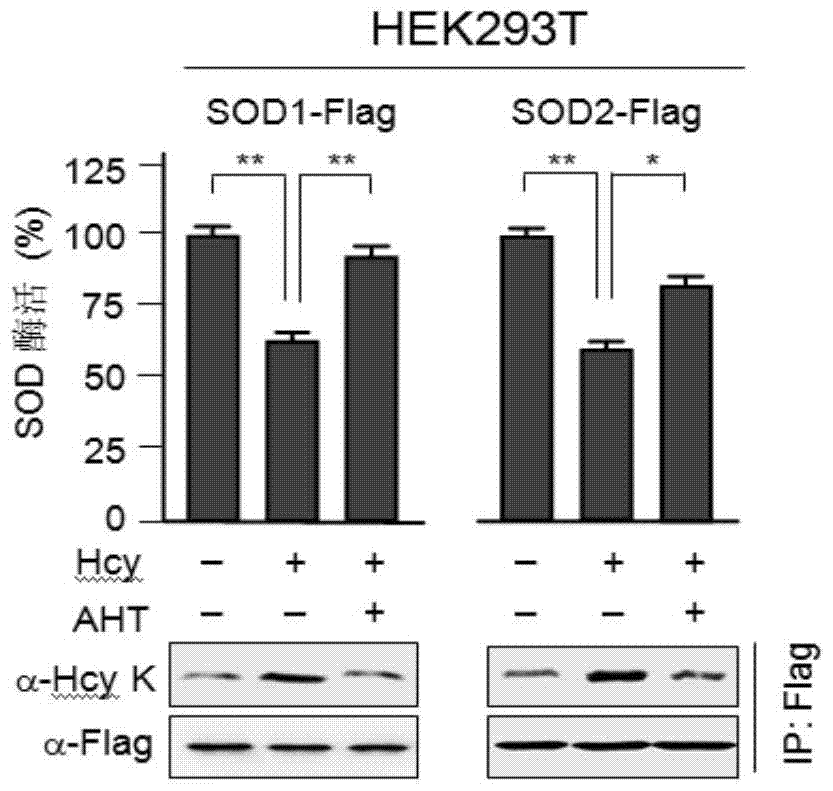
[0056] HTL is the direct precursor of N-homocysteine modification, and AHT, as an inhibitor of HTL production, can reduce the homocysteine modification of total protein in cells, and can inactivate Hcy for total protein homocysteine in cells The added effect of modification.
[0057] Selected cells: HEK293T and NIH3T3, culture conditions: DMEM containing 10% calf serum, 37°C, 5% CO 2 .
[0058] Drug treatment: when the cells were cultured to a confluence of about 80%, AHT was added to the culture medium, and at the same time, Hcy at a uniform concentration was added to both the AHT treatment group and the blank control group to simulate hyperhomocysteinemia. The treatment conditions are as follows surface:
[0059]
[0060] Sample collection and detection: After treating cells with Hcy for 4 hours, wash the cells with PBS 1-2 times, add 1×SDS loading buffer to collect ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com