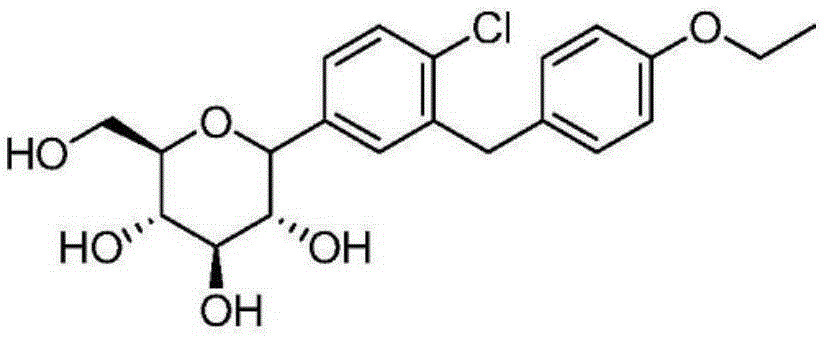
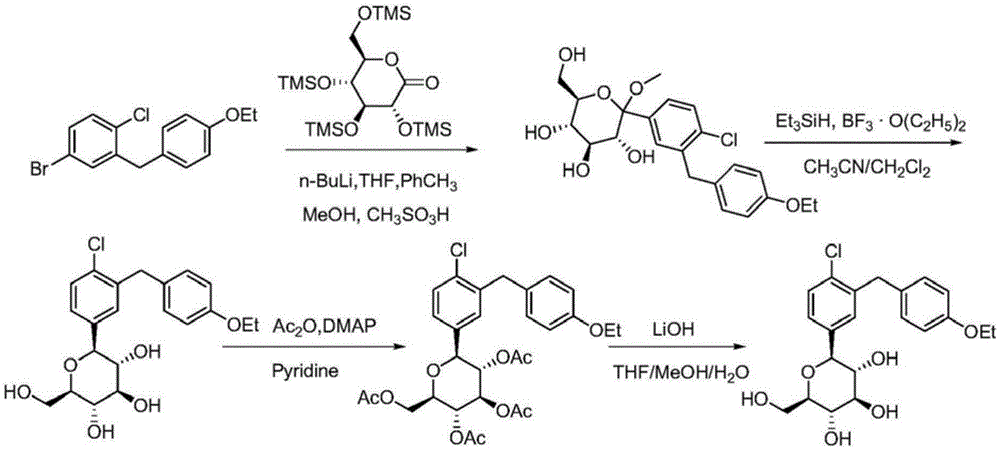
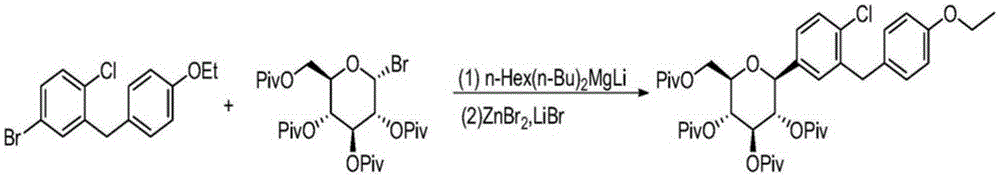Preparation method for dapagliflozin
A technology for Grignard reagents and compounds, applied in the field of preparation of dapagliflozin, can solve the problems to be improved, shorten the reaction process steps, etc., and achieve the effects of saving production time and labor cost, shortening reaction time, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the synthesis of compound II
[0042] Dissolve 10.12g (90.8mmol) Mscl in 250ml CH 2 Cl 2 In, add 20.42g (100.5mmol) fresh Ag 2 O, after stirring at room temperature for several minutes, 10.26g (70mmol) of compound I and 3.5g (20.1mmol) of KI were added successively, and stirred at room temperature for 6h. The progress of the reaction was detected by TLC, after the reaction was completed, it was filtered under reduced pressure, and the residue was washed with CH 2 Cl 2 (50ml×3) wash. The filtrate was concentrated to a small volume and separated by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 5:1) was purified to obtain 10.77g (60.86mmol) of compound II with a yield of 98% and a purity of 99.9%.
Embodiment 2
[0043] Embodiment 2: the synthesis of compound II
[0044] Dissolve 10.87g (90.8mmol) Tscl in 250ml CH 2 Cl 2 In, add 20.42g (100.5mmol) fresh Ag 2 O, after stirring at room temperature for several minutes, 10.26g (70mmol) of compound I and 3.5g (20.1mmol) of KI were added successively, and stirred at room temperature for 6h. The progress of the reaction was detected by TLC, after the reaction was completed, it was filtered under reduced pressure, and the residue was washed with CH2 Cl 2 (50ml×3) wash. The filtrate was concentrated to a small volume and separated by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 5:1) was purified to obtain 20.27g (60.79mmol) of compound II with a yield of 97% and a purity of 99.7%.
Embodiment 3
[0045] Embodiment 3: the synthesis of compound IV
[0046] Add 4-bromo-1-chloro-2-(4-ethoxybenzyl)benzene (40.9g, 150mmol) and 100mL tetrahydrofuran into a 250mL conical flask, stir and cool down to -5~0℃, slowly add n-butylmagnesium chloride dropwise (80mL, 2mol / L), and the system was stirred at 0°C for 2h. Add 10.77g (60.86mmol) compound II (R=-MS), 10.81g (60.94mmol) anhydrous tin tetrachloride and 100ml 2-methyltetrahydrofuran to another 300ml conical flask, and cool the system to 5°C. Slowly add the Grignard reagent in the previous 250ml bottle dropwise, and drop it in about 40 minutes. Return the system to room temperature, keep stirring for 1 hour, quench the system with 1N hydrochloric acid aqueous solution, extract the organic phase with ethyl acetate, wash with saturated brine, and concentrate. Column chromatography (PE / EA=3 / 1) yielded 30.83 g (60.65 mmol) of compound IV with a yield of 97% and a purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com