Reutilization method of by-product ammonium chloride from ammonia process to produce magnesium hydroxide
A technology of magnesium hydroxide and ammonium chloride, applied in organic chemistry and other directions, can solve the problems of low recycling rate, difficulty in compressing magnesium hydroxide, and high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
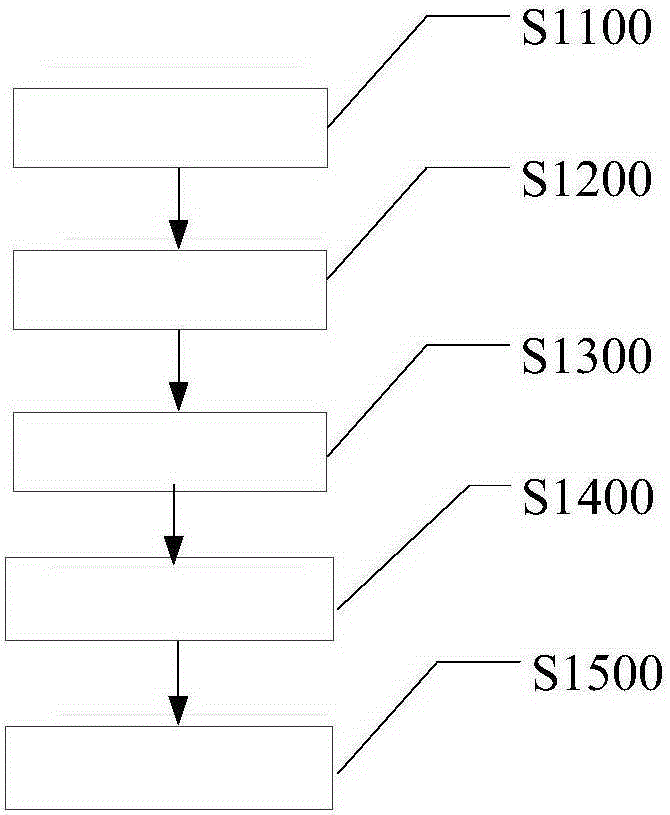
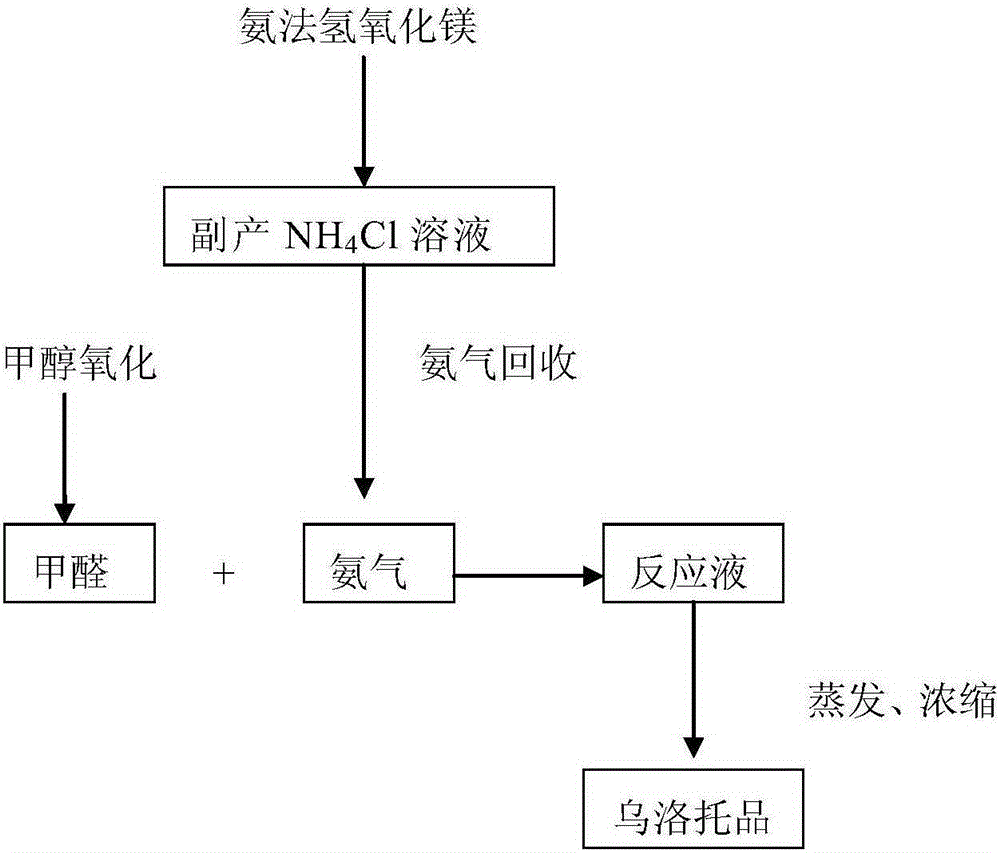
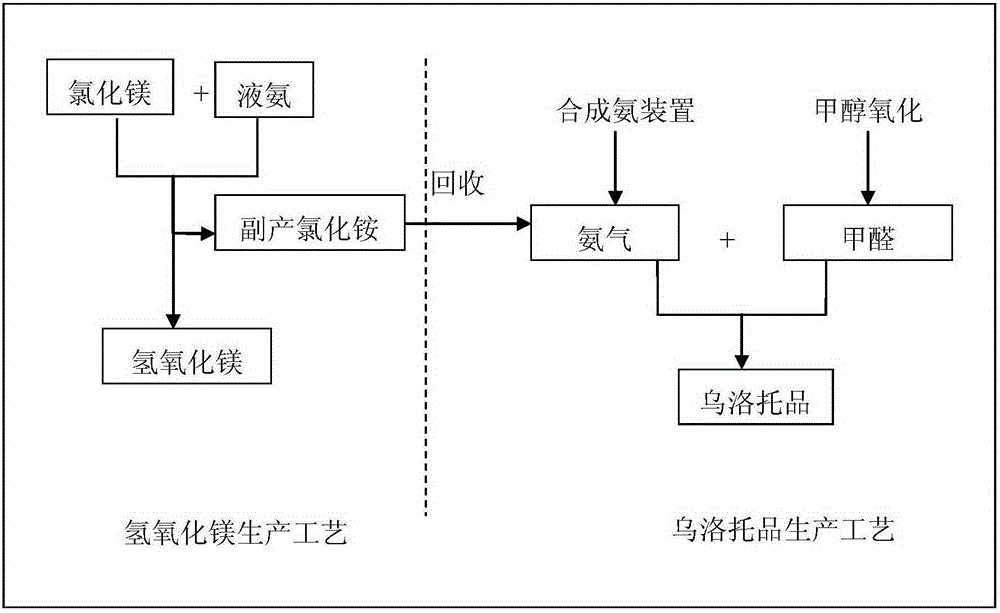
[0033] like figure 1 As shown, first enter into S1100 collection step: collect the by-product ammonium chloride generated by the reaction of magnesium chloride solution and liquid ammonia. Then enter step S1200 to distill ammonia: the calcium oxide and the ammonium chloride collected in step S1100 are used to distill ammonia to produce ammonia gas. In this ammonia distillation step, the stirring speed is 90 rpm, the temperature is 30° C., the pressure in the ammonia distillation kettle is maintained at 50 Pa, and the ammonia distillation time is controlled at 85 minutes. Then enter the condensation reaction step S1300: react the ammonia gas prepared in step S1200 with formaldehyde to form a reaction liquid. Wherein, the reaction temperature is 50° C., the reaction pressure is 0.2 MPa, and the pH value of the reaction solution is controlled at 8. Then enter into S1400 concentration step: evaporate the reaction liquid to form a high-concentration reaction liquid. In this step...
Embodiment 2
[0035] like figure 1 As shown, first enter into S1100 collection step: collect the by-product ammonium chloride generated by the reaction of magnesium chloride solution and liquid ammonia. Then enter step S1200 for distilling ammonia: the calcium chloride and the ammonium chloride collected in step S1100 are used to distill ammonia to produce ammonia gas. In this ammonia distillation step, the stirring speed is 95 rpm, the temperature is 105° C., the pressure in the ammonia distillation kettle is maintained at 55 kPa, and the ammonia distillation time is controlled at 90 minutes. Then enter the condensation reaction step S1300: react the ammonia gas prepared in step S1200 with formaldehyde to form a reaction liquid. Wherein, the reaction temperature is 70° C., the reaction pressure is 0.25 MPa, and the pH value of the reaction solution is controlled at 9. Then enter into S1400 concentration step: evaporate the reaction liquid to form a high-concentration reaction liquid. In...
Embodiment 3
[0037] like figure 1As shown, first enter into S1100 collection step: collect the by-product ammonium chloride generated by the reaction of magnesium chloride solution and liquid ammonia. Then enter step S1200 to distill ammonia: the calcium oxide and the ammonium chloride collected in step S1100 are used to distill ammonia to produce ammonia gas. In this ammonia distillation step, the stirring speed is 92 rpm, the temperature is 70° C., the pressure in the ammonia distillation kettle is maintained at 52 kPa, and the ammonia distillation time is controlled at 88 minutes. Then enter the condensation reaction step S1300: react the ammonia gas prepared in step S1200 with formaldehyde to form a reaction liquid. Wherein, the reaction temperature is 60° C., the reaction pressure is 0.23 MPa, and the pH value of the reaction solution is controlled at 8.5. Then enter into S1400 concentration step: evaporate the reaction liquid to form a high-concentration reaction liquid. In this s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com