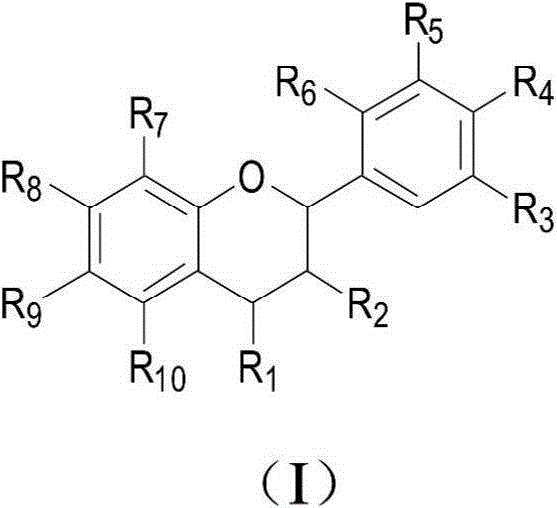
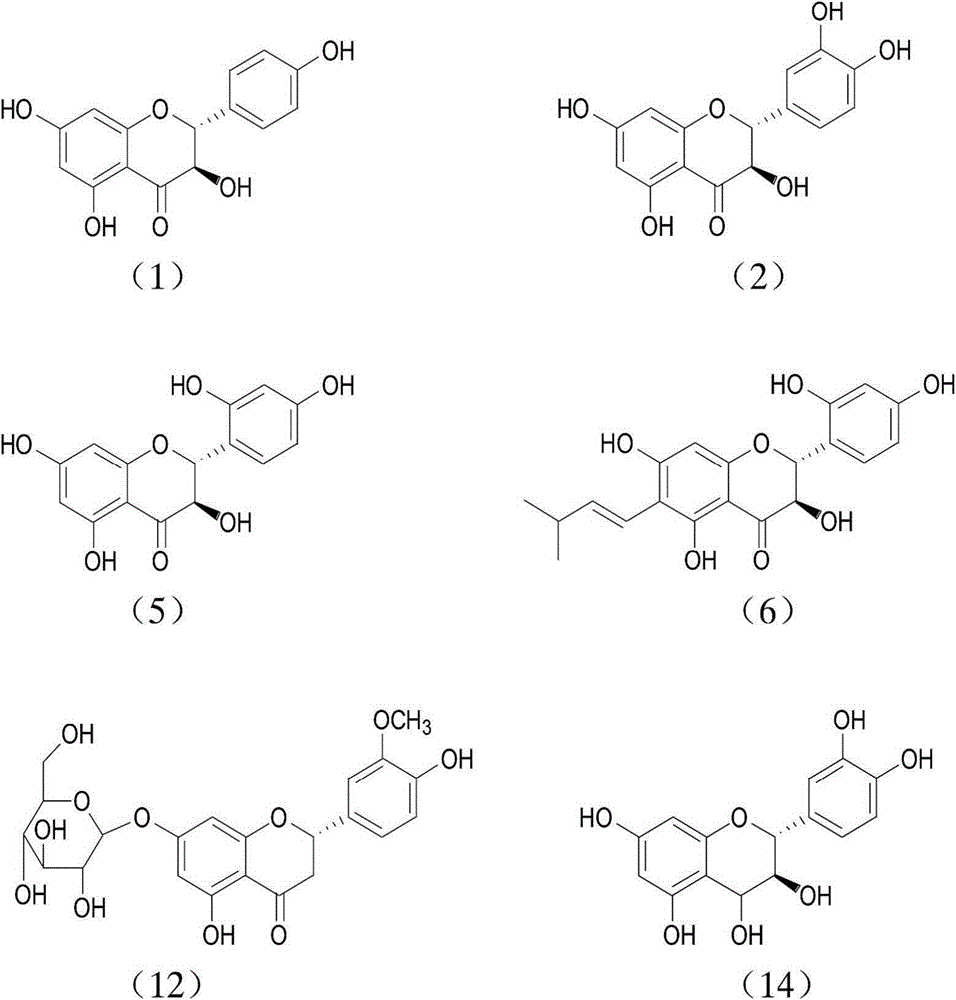
Novel application of benzopyran derivative in preparation of drug for treating hyperuricaemia
A benzopyran derivative, a technology for hyperuricemia, applied in the field of pharmaceutical compositions for treating hyperuricemia and gout, can solve problems such as large toxic and side effects, achieve no toxic and side effects, reduce serum uric acid levels, The effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
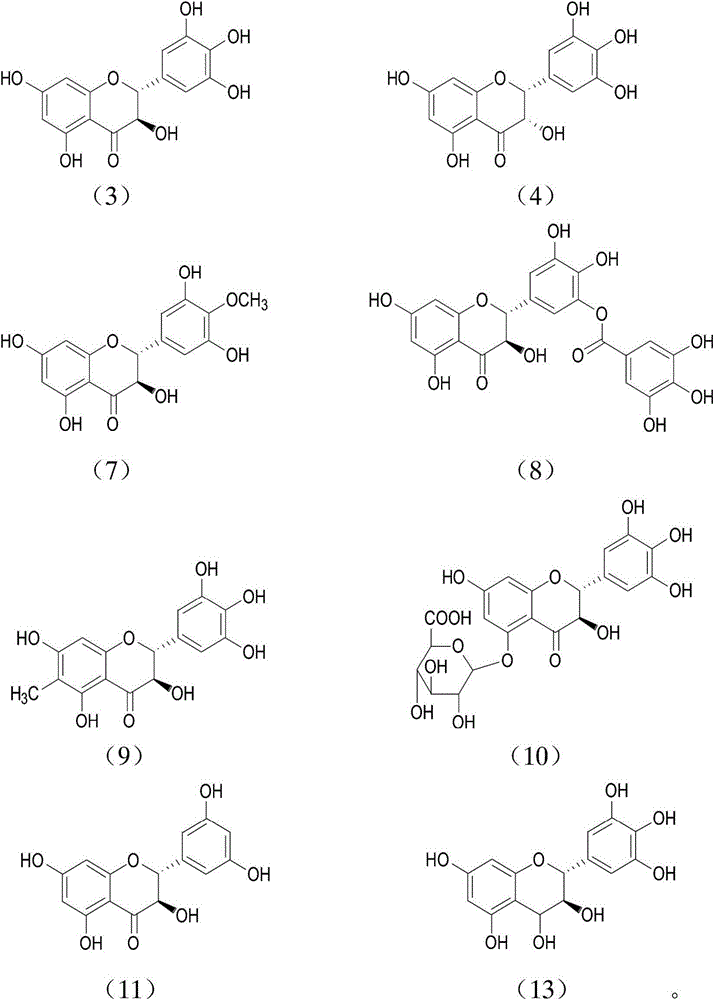
[0102] Embodiment 1: Preparation and characterization of compound 4 of the present invention
[0103] Dissolve compound 3 in distilled water, prepare a solution with a mass percent concentration of 3%, adjust the pH of the solution to 4.0-6.0, put the reaction solution into an autoclave, and react at 120-130°C for 40-60 minute. After the reaction solution is cooled to room temperature, it is separated by D101 macroporous adsorption resin, eluted with distilled water and 70% ethanol in sequence, the eluate is concentrated and left to crystallize, and the collected crystal is compound 4 of the present invention, which is detected and characterized as follows:
[0104] 1 HNMR (DMSO-d 6 , 400MHz) δ: 11.90(1H, s), 10.78(1H, s), 6.16(1H, d, J=5.1Hz), 5.27(1H, brs), 4.02(1H, brd), 5.90(1H, brs ), 5.85 (1H, brs), 6.44 (2H, s), 8.20 (1H, brs); 13 C-NMR (DMSO-d 6 , 100MHz) δppm: 81.1, 71.0, 195.6, 163.9, 95.8, 166.7, 94.9, 162.7, 100.3, 126.1, 106.6, 145.5, 132.9. It can be seen ...
Embodiment 2
[0105] Embodiment 2: Preparation and characterization of compound 13 of the present invention
[0106] Dissolve compound 3 (500 mg) in 50 ml of ethanol, stir and add 200 mg of NaBH4, react at room temperature for 1 hour. Immediately after the reaction was completed, 50ml of 0.1N hydrochloric acid and 150ml of water were added, and the mixture was extracted with 150ml of ethyl acetate. The organic phase was evaporated to dryness, the residue was dissolved in an appropriate amount of water, and then separated on an ODS reversed-phase silica gel column, and gradient elution was carried out with ethanol-water, and the compound 13 of the present invention was isolated, and its detection and characterization data were as follows:
[0107] 1 H-NMR (DMSO-d 6 , 400MHz) δppm: 4.45 (1H, dd, J = 11.0, 3.5Hz), 4.56 (1H, dd, J = 11.0, 3.5Hz), 4.92 (1H, d, J = 11.0Hz), 5.88 (1H, d , J=1.2Hz), 5.92(1H, d, J=1.2Hz), 6.42(2H, s), 11.90(1H, s); 13 C-NMR (DMSO-d 6 , 100MHz) δppm: 72.34, 74.3...
Embodiment 3
[0108] Example 3: Extraction and characterization of compounds 7, 8, and 10 of the present invention
[0109] Take 1 kg of young leaves of vine tea (Ampelopsis grossedentata), and extract with 8 times the amount of 70% ethanol under reflux. The extract was concentrated to dryness in vacuo, the residue was suspended in water, and extracted with petroleum ether, ethyl acetate, and n-butanol in sequence. The ethyl acetate extract was separated by column chromatography repeatedly with forward silica gel, the eluent was chloroform-methanol system, the eluent was concentrated, and then further separated with ODS reversed-phase silica gel, and the gradient was carried out with ethanol-water as the eluent After elution, compound 7, compound 8 and compound 10 of the present invention can be obtained respectively; the detection and characterization data are as follows:
[0110] Compound 7: 1 H-NMR (DMSO-d 6 , 400MHz) δppm: 4.40 (1H, d, J = 11.0Hz), 4.92 (1H, d, J = 11.0Hz), 5.88 (1H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com