Eye mask for treating diabetic macular edema
A technology for macular edema and diabetic patients, applied in the field of medical preparations, can solve problems such as difficulty in exerting drug efficacy, difficulty in breaking through the blood-retinal barrier, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
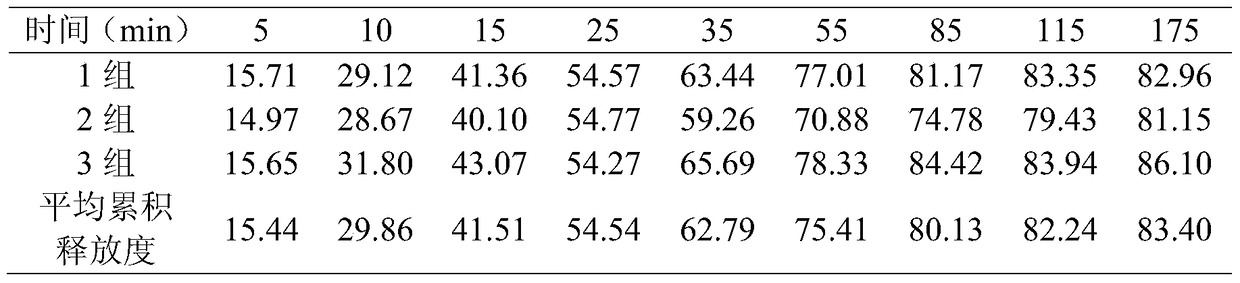
[0028] An eye patch comprises medicine and a polylactic acid film loaded with medicine, the medicine is aspirin acceptable to diabetic retinopathy patients, the film is polylactic acid film, and the mass ratio of polylactic acid to aspirin is 8 / 3.
[0029] This eye patch is accomplished through the following steps:
[0030] Step 1: Ingredients: Weigh an appropriate amount of polylactic acid and aspirin respectively, crush and sieve, dry in vacuum, and place them in an airtight container.
[0031] Step 2: Mixing: Add polylactic acid to ethyl acetate, stir under airtight conditions to completely dissolve it into solution A, and adjust the pH of the solution to 6.3;
[0032] Step 3: Polymerization: add one of processed aspirin, 2,5-dihydroxybenzenesulfonate calcium hydrate, and dexamethasone to solution A, add a certain proportion of glycerin, dissolve it with ultrasonic waves, and react for 15 minutes. Ultrasonic degassing for 40 minutes to form drug membrane slurry B.
[0033...
Embodiment 2
[0037] An eye patch, comprising a drug and a drug-loaded polylactic acid film, the drug is 2,5-dihydroxybenzenesulfonate calcium-hydrate acceptable to diabetic retinopathy patients, the film is a polylactic acid film, the The mass ratio of polylactic acid to 2,5-dihydroxybenzenesulfonate calcium-hydrate is 2 / 1.
[0038] This eye patch is accomplished through the following steps:
[0039] Step 1: Ingredients: Weigh appropriate amounts of polylactic acid and calcium 2,5-dihydroxybenzenesulfonate-hydrate, crush and sieve, dry in vacuum, and place them in an airtight container.
[0040] Step 2: Mixing: Add polylactic acid to ethyl acetate, stir under airtight conditions to completely dissolve it into solution A, and adjust the pH of the solution to 6.8;
[0041] Step 3: Polymerization: Add processed calcium 2,5-dihydroxybenzenesulfonate-hydrate to solution A, add a certain proportion of glycerin, heat to 80°C with steam, dissolve, react for 20 minutes, water bath or ultrasonic de...
Embodiment 3
[0046] An eye patch, comprising a drug and a drug-loaded polylactic acid film, the drug is acceptable dexamethasone for diabetic retinopathy patients, the film is a polylactic acid film, one of the polylactic acid and dexamethasone The mass ratio of species is 80 / 1.
[0047] This eye patch is accomplished through the following steps:
[0048] Step 1: Ingredients: Weigh an appropriate amount of polylactic acid and dexamethasone, pulverize, sieve, dry in vacuum, and place in an airtight container.
[0049] Step 2: Mixing: Add polylactic acid to ethyl acetate, stir under airtight conditions to completely dissolve it into solution A, and adjust the pH of the solution to 7.5;
[0050] Step 3: Polymerization: add one of processed aspirin, 2,5-dihydroxybenzenesulfonate calcium-hydrate, and dexamethasone to solution A, add a certain proportion of glycerin, dissolve it with ultrasonic waves, and react for 20 minutes. Degassing in a water bath for 40 minutes to form drug membrane slur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com