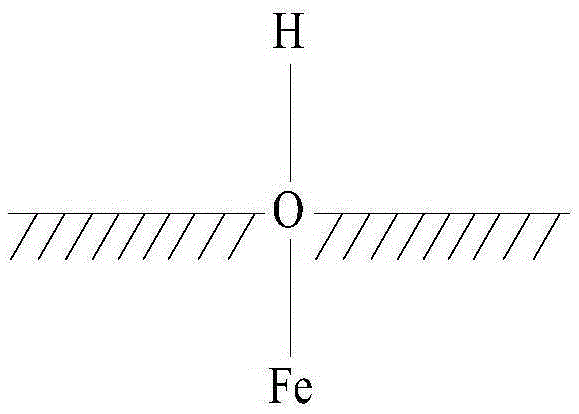Cesium ion adsorbent and preparation method thereof
An adsorbent and cesium ion technology, applied in the field of cesium ion adsorbent and its preparation, can solve the problems of difficult to obtain a chromatographic column, high price of extraction agent, complicated steps, etc., achieve strong cesium ion adsorption selectivity, and improve preparation efficiency , the effect of rapid solid-liquid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The present embodiment provides a cesium ion adsorbent, which includes hydroxylated ferric oxide and (+)-(18-crown which is linked to the hydroxylated ferric oxide by hydrogen bonding with a mass ratio of 38:1. -6)-2,3,11,12-tetracarboxylic acid.
[0032] In the present embodiment, the specific surface area of the hydroxylated iron tetroxide in the cesium ion adsorbent is 213m 2 / g.
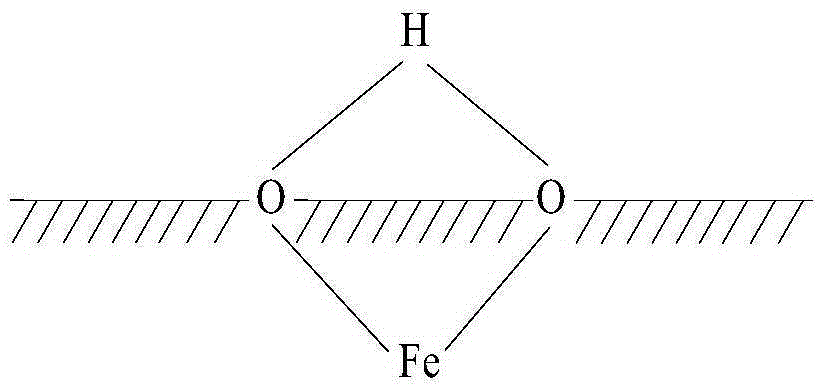
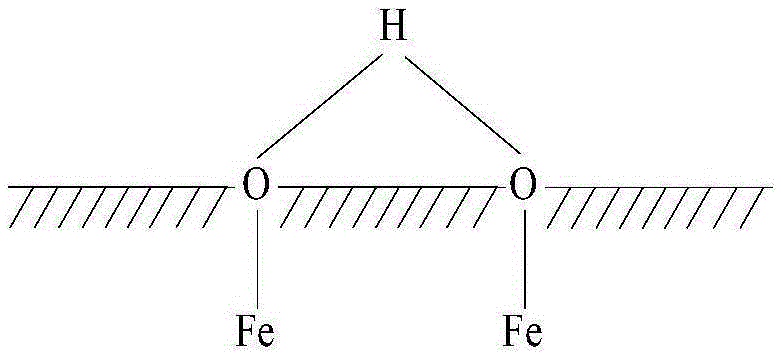
[0033] It is worth noting that, in this embodiment, the hydroxylated Fe3O4 means that several hydroxyl groups are attached to the surface of the Fe3O4 particles, but not every molecule of Fe3O4 is attached with a hydroxyl group. Such as Figure 1 to Figure 3 As shown, the structure of the hydroxylated ferric oxide includes three kinds; Figure 1 to Figure 3 In , the shaded part represents the interior of Fe3O4 particles, from Figure 1 to Figure 3 It can be clearly seen that the O atom is located on the surface of the ferric oxide particle, and the O atom is also connected with an H ...
Embodiment 2
[0049] In the description of Embodiment 2, the similarities with Embodiment 1 will not be repeated here, and only the differences with Embodiment 1 will be described. The difference between embodiment 2 and embodiment 1 is that the cesium ion adsorbent in embodiment 2 includes hydroxylated ferric oxide and 4-aminobenzo-18-crown-6 with a mass ratio of 42:1, and The specific surface area of the hydroxylated ferric oxide is 194m 2 / g.
[0050] The difference between the preparation method of the cesium ion adsorbent in this example and the preparation method in Example 1 is that in step 1, 1.3mmol iron source, 7mL ethylene glycol and 25mL deionized water are mixed and stirred for 0.5h , obtain the third mixture; Utilize 0.6mol / L aqueous ammonia solution to adjust the pH value of the third mixture to 9.0; keep the third mixture with the pH value of 9.0 in the hydrothermal reactor with a temperature rise of 5° C. / min Raise the temperature to 180°C, and keep it at this temperatu...
Embodiment 3
[0059] In the description of Embodiment 3, the similarities with Embodiment 1 will not be repeated here, and only the differences with Embodiment 1 will be described. The difference between embodiment 3 and embodiment 1 is that the cesium ion adsorbent in embodiment 3 includes hydroxylated ferric oxide and 4'-carboxybenzo-18-crown-6 with a mass ratio of 34:1, And the specific surface area of the hydroxylated ferric oxide is 233m 2 / g.
[0060] The difference between the preparation method of the cesium ion adsorbent in this example and the preparation method in Example 1 is that in step 1, 0.87mmol iron source, 3mL glycerol and 30mL deionized water are mixed and stirred for 0.5h , obtain the third mixture; Utilize the aqueous ammonia solution of 0.4mol / L to adjust the pH value of the third mixture to 9.0; all the other referring to step one in Example 1, the obtained specific surface area is 233m 2 / g of hydroxylated ferric oxide.
[0061] In step 2, the hydroxylated ferr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com