Cesium ion adsorbing agent and preparing method thereof
A technology of adsorbent and cesium ion, which is applied in the field of cesium ion adsorbent and its preparation, can solve the problems of difficult to prepare chromatographic column, large exchange potential, complicated steps, etc., and achieve strong cesium ion adsorption selectivity, stable structure and properties , The effect of rapid solid-liquid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment provides a cesium ion adsorbent, which includes carboxylated ferric oxide and 2-aminomethyl-18-crown-6 condensed and linked with the carboxylated ferric oxide through amidation.
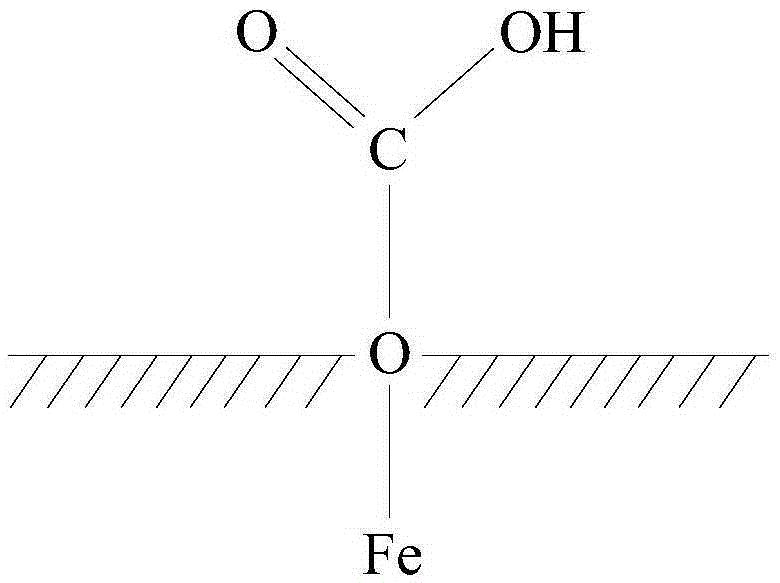
[0028] It is worth noting that, in this embodiment, the carboxylation of ferric oxide refers to having several carboxyl groups on the surface of ferric oxide particles, rather than carboxyl groups attached to each ferric oxide molecule. Such as figure 1 Shown is the structural representation of described carboxylated ferric oxide; figure 1 In , the shaded part represents the interior of Fe3O4 particles, from figure 1 It can be clearly seen that the O atom is located on the surface of the ferric oxide particle, and the O atom is also connected with COOH, that is to say, the surface of the ferric oxide particle has been carboxylated, and the carboxylated ferric oxide particle has been obtained. Iron, and the carboxyl group can be condensed with the aminated crown ether derivati...
Embodiment 2
[0043] In the description of Embodiment 2, the similarities with Embodiment 1 will not be repeated here, and only the differences with Embodiment 1 will be described. The difference between embodiment 2 and embodiment 1 is that the cesium ion adsorbent in embodiment 2 includes carboxylated ferric oxide and the 4-amino group connected to the carboxylated ferric oxide by amidation condensation Benzo-18-crown-6.
[0044] The difference between the preparation method of the cesium ion adsorbent of the present embodiment and the preparation method in Example 1 is that in step one, the ratio of 1 g of ferric citrate and citric acid is 1:50 according to the ratio of the amount of substance Mix to obtain a third mixture; add deionized water to the third mixture to make 80 mL of the first solution, place the first solution in a hydrothermal reactor and heat it up to 220°C, and keep it at this temperature. Heat reaction for 24 hours to obtain the fourth mixture; for the rest, refer to ...
Embodiment 3
[0053] In the description of Embodiment 3, the similarities with Embodiment 1 will not be repeated here, and only the differences with Embodiment 1 will be described. The difference between embodiment 3 and embodiment 1 is that the cesium ion adsorbent in embodiment 3 includes carboxylated ferric oxide and 4-amino di Benzo-18-crown-6.
[0054] The difference between the preparation method of the cesium ion adsorbent of the present embodiment and the preparation method in Example 1 is that in step one, the ratio of 1g ferrous ammonium sulfate and oleic acid according to the amount of substance is 1:10 Mix according to the ratio to obtain the third mixture; add deionized water to the third mixture to make 80mL of the first solution, place the first solution in a hydrothermal reaction kettle and raise the temperature to 220°C, and keep it at this temperature Undergo hydrothermal reaction for 24 hours to obtain the fourth mixture; for the rest, refer to step 1 in Example 1 to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com