2-cyclohexene-1-ketone preparation method
A technology of cyclohexene and alkyl vinyl ether, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carbon-based compounds, etc., can solve problems such as large equipment investment, achieve low environmental pollution and strong industrial application value , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
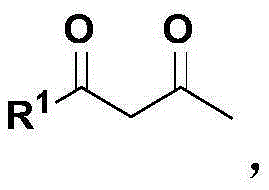
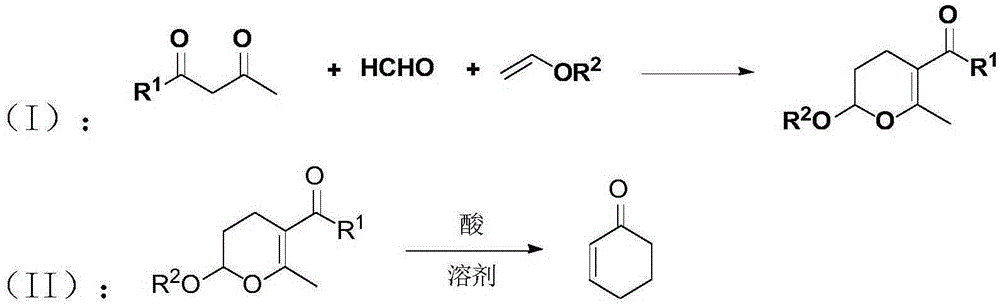
[0024] The preparation method of 2-cyclohexen-1-one among the present invention is mainly realized through the following steps:
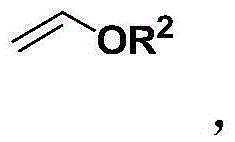
[0025] Step (1): Put 1,3-dicarbonyl compound, alkyl vinyl ether and formaldehyde into a reactor equipped with magnetic stirring, wherein the molar ratio of 1,3-dicarbonyl compound to alkyl vinyl ether is 3 : 1 to 1: 3, the molar ratio of 1,3-dicarbonyl compound to formaldehyde is 1: 1 to 1: 3, the above materials are mixed and reacted at 50 to 110 ° C for 1 to 8 hours, and after the reaction is completed, they are distilled under reduced pressure to obtain 2-Alkoxy-3,4-dihydropyran derivatives.
[0026] Step (2): Put the 2-alkoxy-3,4-dihydropyran derivative obtained in step (1), acid and solvent into a reactor equipped with magnetic stirring to obtain a mixed solution; wherein, the acid and 2 - The molar ratio of alkoxy-3,4-dihydropyran derivatives is 1:1 to 8:1, and the ratio of solvent volume to 2-alkoxy-3,4-dihydropyran derivatives is 0.5 to 5 ...
Embodiment 1
[0029] Embodiment 1: Ethyl vinyl ether, formaldehyde and methyl acetoacetate prepare cyclohexenone
[0030]
[0031] Take 144.2 g (2.0 mol) of ethyl vinyl ether, 30.0 g (1.0 mol) of paraformaldehyde, and 116.1 g (1.0 mol) of methyl acetoacetate, mix them in methanol (500 ml), and stir at 50° C. for 5 hours. After the reaction was completed, the solvent and unreacted raw material ethyl vinyl ether were distilled off, and then distilled under reduced pressure to obtain 6-methyl-2-ethoxyl-5-carboxymethyl-3,4-dihydropyran 160.1g ( 0.8mol). 1 HNMR (400MHz, CDCl 3 ,TMS,25℃)δ=5.10–4.97(m,1H),3.92–3.79(m,1H),3.69(s,3H),3.66–3.54(m,1H),2.42–2.28(m,2H) ,2.24(s,3H),1.90–1.82(m,1H),1.82–1.70(m,1H),1.22ppm(td,J=7.1,0.8Hz,3H). 13 CNMR (100MHz, CDCl 3 ,25℃)δ=168.9,162.0,102.0,98.0,64.3,51.1,26.3,20.0,18.0,15.2ppm.IR(KBr):v=2976,2947,1711,1630,1436,1379,1276,1185, 1120,1079,1013,971,861,769cm -1 .HRMS(TOF,ESI):m / zcalcdforC 10 h 17 o 4 ,[M+H] + 201.1127, found 201.1122. 160.1 g (...
Embodiment 2
[0032] Embodiment 2: Butyl vinyl ether, formaldehyde and ethyl acetoacetate prepare cyclohexenone
[0033]
[0034] Take 300.2 g (3.0 mol) of butyl vinyl ether, 162.1 g (2.0 mol) of formaldehyde aqueous solution, and 130.1 g (1.0 mol) of ethyl acetoacetate, mix and react at 110° C. for 6 hours. After the reaction was completed, the unreacted raw material butyl vinyl ether was distilled off, and then distilled under reduced pressure to obtain 171.9 g (0.71 mol ). 1 HNMR (600MHz, CDCl 3 ,TMS,25℃)δ=5.02(d,J=1.0Hz,1H),4.21–4.10(m,2H),3.80(dt,J=8.5,6.7Hz,1H),3.59–3.48(m,1H ),2.42–2.27(m,2H),2.24(s,3H),1.88–1.83(m,1H),1.81–1.71(m,1H),1.64–1.51(m,2H),1.40–1.33(m ,2H),1.28(t,J=7.1Hz,3H),0.91ppm(t,J=7.4Hz,3H). 13 CNMR (150MHz, CDCl 3 , 25°C) δ=168.32, 161.51, 102.04, 97.92, 68.40, 59.57, 31.64, 26.11, 19.89, 19.18, 17.81, 14.35, 13.7ppm. 171.9 g (0.71 mol) of the obtained dihydropyran derivative, 547 g (4.5 mol) of hydrochloric acid aqueous solution (30 wt %) were mixed in butan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com