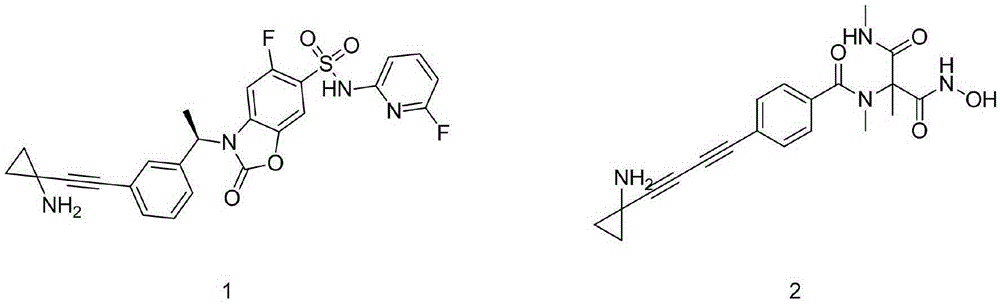
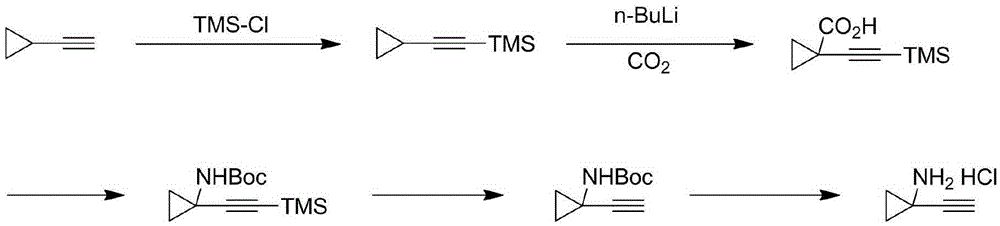
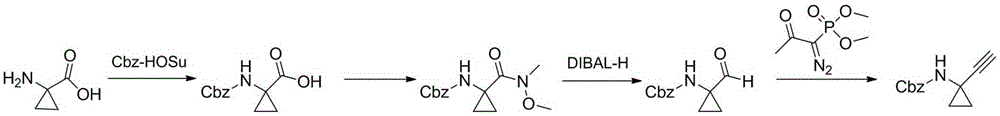
Technological synthesis method of 1-amino cyclopropyl acetylene
A synthesis method and amino ring technology, which are applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of carbamic acid derivatives, etc., can solve the problems of cost increase, expensive dimethyl phosphonate, unfavorable market competition, etc., and achieve cost reduction. The effect of low cost, high safety, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] Add compound 1A (7g, 0.037mol, 1eq) and dichloromethane (50mL) to 500mL there-necked flask, add 4-OH-TEMPO (0.06g, 0.37mmol, 0.01eq), add saturated NaHCO 3 Aqueous solution (200mL) was cooled to 0°C, 10% NaClO aqueous solution (50g, 0.067mol, 1.8eq) was added dropwise, the temperature was naturally raised to room temperature, and reacted for 1h. Add 100 mL of water, extract with dichloromethane (150 mL*2), combine the organic phases, and wash once with water (200 mL), concentrate the organic phase to obtain compound 2A as a light yellow solid (6.3 g, yield: 91%, purity: 94.2% )
[0043] 1 HNMR (400MHz, CDCl 3 ): δ=9.181(s, 1H), 5.142(t, 1H), 1.483(m, 2H), 1.413(s, 9H), 1.270(m, 2H)ppm; ESI / MS: m / z=186( M+H)+.
Embodiment 2
[0045]
[0046] Add triphenylphosphine (45.4g, 0.17mol, 4eq) and DCM (255g) into a 250mL three-necked flask, cool the reaction solution to 0-5°C, add CBr 4 (28.6g, 0.086mol, 2eq), the color of the solution changed from colorless to red, and a solid precipitated out after 20min. Compound 2A (8 g, 0.043 mol, 1 eq) was added in 4 batches, the exotherm was obvious, and the reaction was carried out at 0°C for 0.5 h. The solid was removed by filtration, concentrated, brushed with silica gel, and the eluent was concentrated to obtain 13.7 g of a light yellow solid.
[0047] 1 HNMR (400MHz, CDCl 3):δ=6.687(s,1H), 1.453(s,9H), 1.288(m,2H), 1.253(m,2H)ppm; ESI / MS: m / z=342(M+H)+.
[0048] Add the light yellow solid to THF (270mL), cool down to -78°C, add 2.5Mn-BuLi / hexane solution (53mL, 0.130mol, 3eq) dropwise, react at -78°C for 30min, then slowly raise the temperature to -40°C, React for 30 minutes. Water (100 mL) was slowly added dropwise at -40°C, the temperature was slowly ...
Embodiment 3
[0051]
[0052] Compound 3A (2.9g, 0.016mol, 1eq) and 7.1M HCl / MTBE solution (25mL, 0.178mol, 11eq) were added into a 100mL three-necked flask, and stirred overnight at room temperature. Filtration, the filter cake was rinsed with MTBE (10mL), the solid was collected and dried to obtain compound 4 as a white solid (1.7g, 90%, purity: 99%)
[0053] 1 HNMR (400MHz, CDCl 3 ): δ=9.082(s, 3H), 3.562(s, 1H), 1.331(t, 2H), 1.126(t, 2H) ppm; ESI / MS: m / z=82(M+H)+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com