Preparation method of roxatidine acetate hydrochloride for injection
A technology for roxatidine hydrochloride acetate and injection, which is applied in the field of preparation of roxatidine hydrochloride acetate for injection, can solve problems such as easy hydrolysis, short validity period, and impact on product quality, and achieve favorable preservation and quality Stable and quality-guaranteed effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
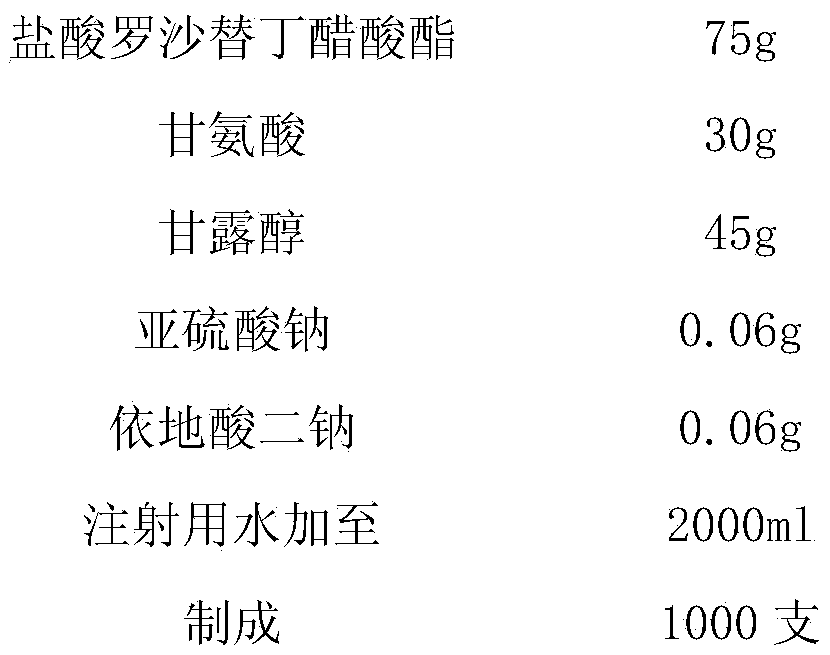
[0017] prescription:
[0018]
[0019] Process:
[0020] (1) Take by weighing roxatidine hydrochloride acetate and mix with glycine, mannitol, sodium sulfite, disodium edetate according to the prescription proportion, add 1000~2000ml of water for injection, stir to dissolve completely, add activated carbon, filter, Decarburization, to obtain system A;
[0021] (2) Add water for injection to the full amount in system A, and stir evenly to obtain system B;
[0022] (4) According to the requirements of aseptic operation in the 100-class laminar flow ultra-clean room, the system B is filtered by ultrafiltration, packed in controlled antibiotic bottles, added with a ventilated rubber stopper, pressed after freeze-drying, and added with an aluminum-plastic composite cover. have to.
Embodiment 2
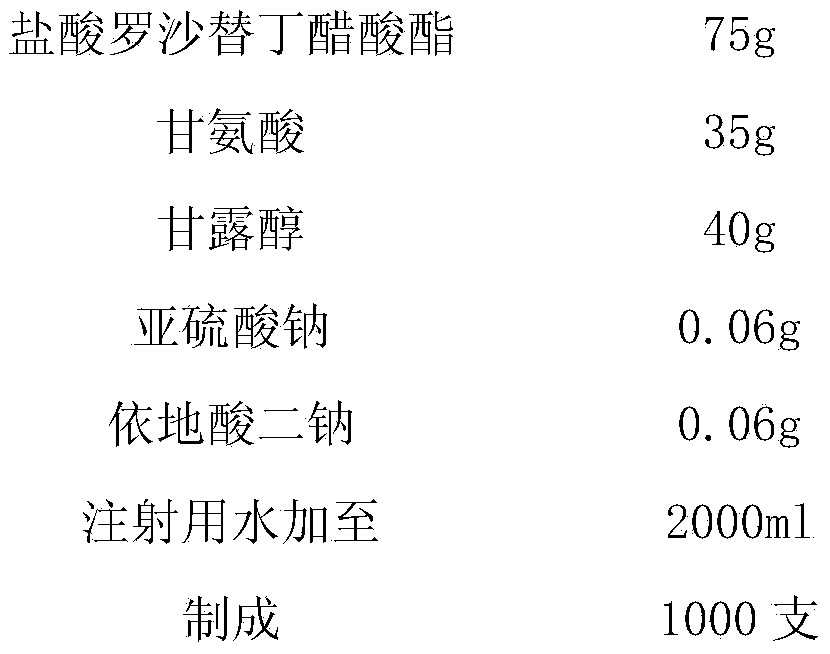
[0024] prescription:
[0025]
[0026] Process:
[0027] (1) Take by weighing roxatidine hydrochloride acetate and mix with glycine, mannitol, sodium sulfite, disodium edetate according to the prescription proportion, add 1000~2000ml of water for injection, stir to dissolve completely, add activated carbon, filter, Decarburization, to obtain system A;
[0028] (2) Add water for injection to the full amount in system A, and stir evenly to obtain system B;
[0029] (4) According to the requirements of aseptic operation in the 100-class laminar flow ultra-clean room, the system B is filtered by ultrafiltration, packed in controlled antibiotic bottles, added with a ventilated rubber stopper, pressed after freeze-drying, and added with an aluminum-plastic composite cover. have to.
Embodiment 3
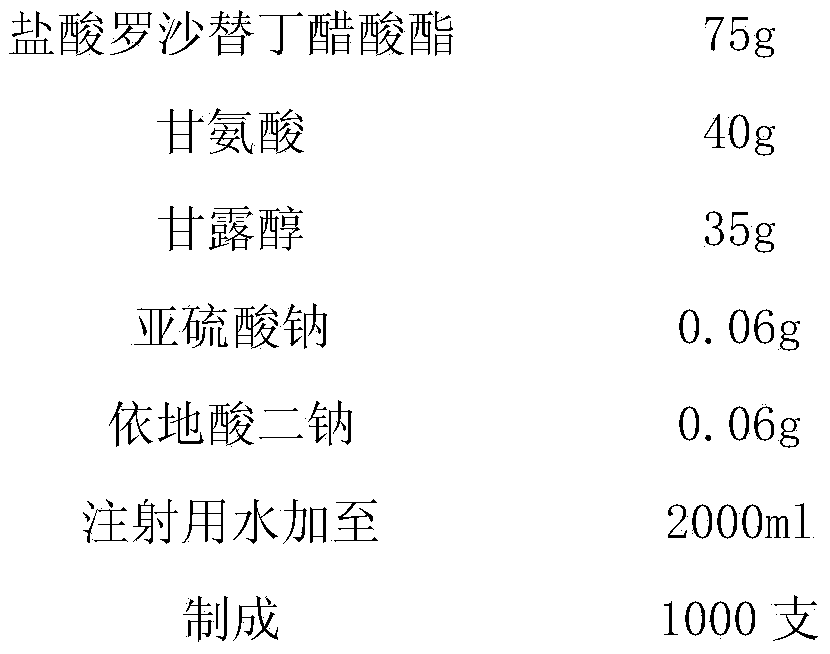
[0031] prescription:
[0032]
[0033] Process:
[0034] (1) Take by weighing roxatidine hydrochloride acetate and mix with glycine, mannitol, sodium sulfite, disodium edetate according to the prescription proportion, add 1000~2000ml of water for injection, stir to dissolve completely, add activated carbon, filter, Decarburization, to obtain system A;
[0035] (2) Add water for injection to the full amount in system A, and stir evenly to obtain system B;
[0036] (4) According to the requirements of aseptic operation in the 100-class laminar flow ultra-clean room, the system B is filtered by ultrafiltration, packed in controlled antibiotic bottles, added with a ventilated rubber stopper, pressed after freeze-drying, and added with an aluminum-plastic composite cover. have to.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com