Four co(ii)-based metal-organic frameworks and their preparation methods and applications
A metal-organic framework and reaction technology, applied in the field of Co-based metal-organic frameworks and their preparation, can solve the problems of poor stability, inability to recycle, and complicated preparation steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of organic ligand L, the specific preparation steps are as follows:
[0068] N 2 Under protection, 2,5-dibromopyridine (2.37g, 10mmol), 1,2,4-triazole (1,66g, 24mmol), cesium carbonate (13.03g, 40mmmol), cuprous iodide (0.76g, 4mmol) was placed in a 100mL three-necked flask, 20mL DMF was used as a solvent, heated to 120°C, followed by TLC, poured into 400mL water after the reaction was completed, left to stand, suction filtered, and after the solid was dried, it was subjected to silica gel column chromatography (dichloromethane: methanol =20:1), 1.49 g of white solid was obtained with a yield of 75%.
[0069]
[0070] Structural characterization of the organic ligand L prepared in this example, its 1 HNMR, IR respectively as Figure 11 , 12 shown.
Embodiment 2
[0071] Embodiment 2: Synthesis of Co-MOF-1
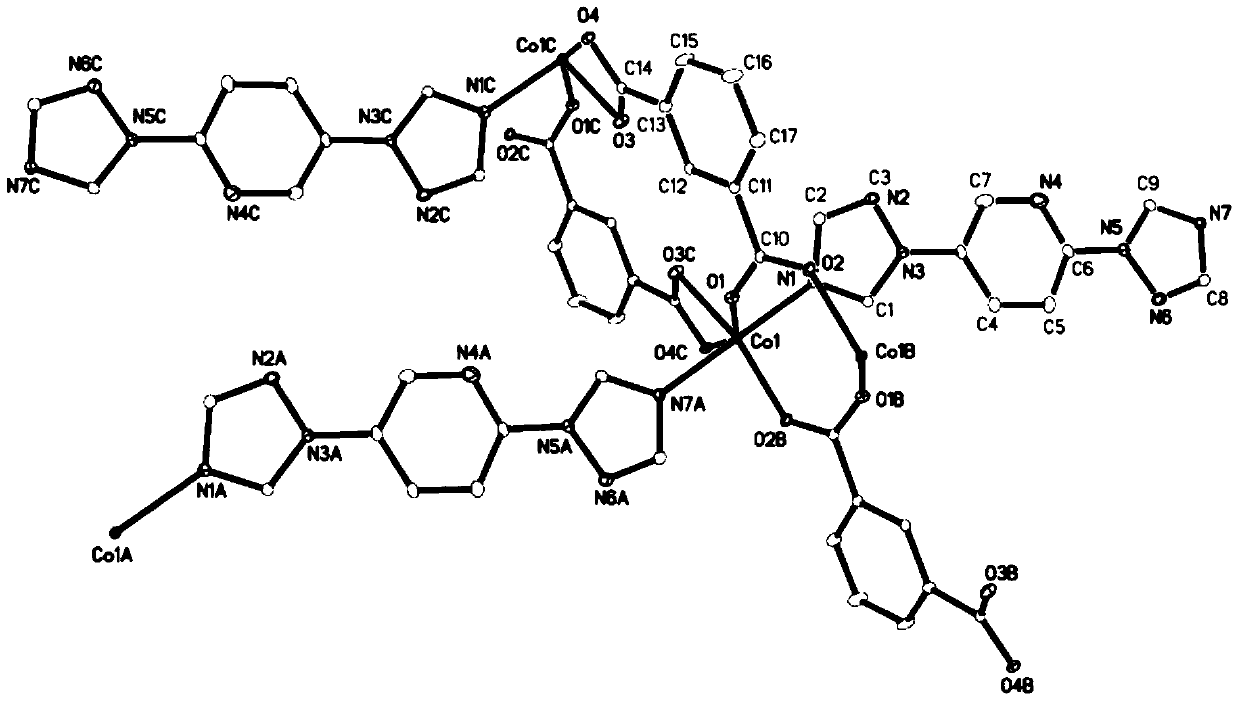
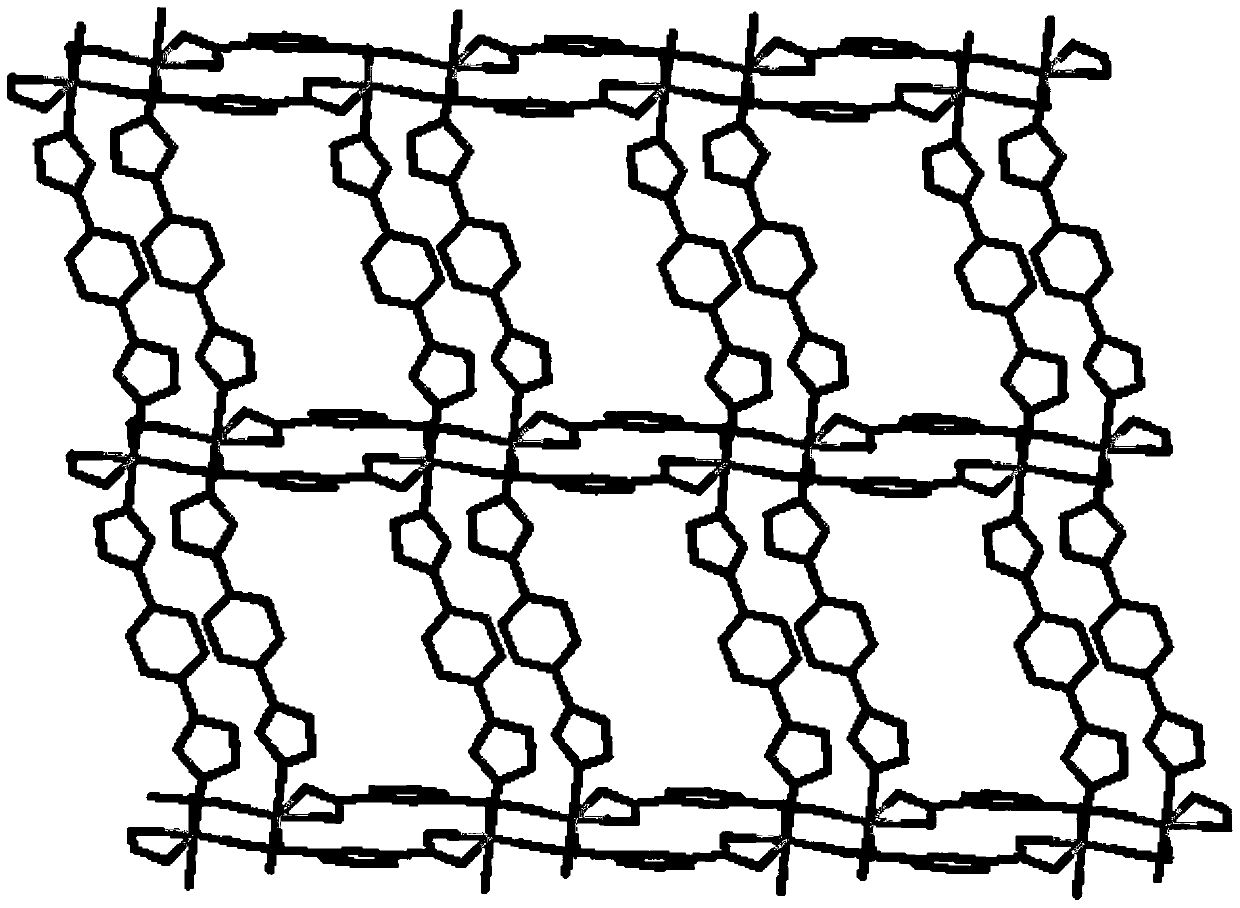
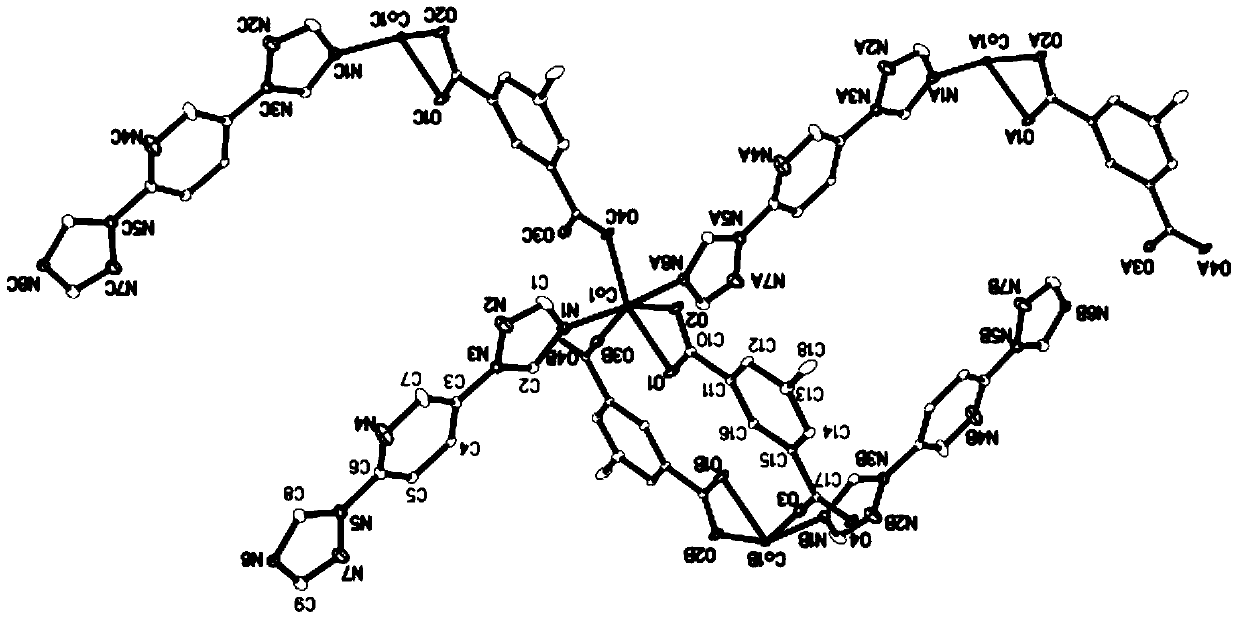
[0072] The L (3.0mg, 0.014mmol) prepared in Example 1, isophthalic acid (2.5mg, 0.015mmol) Co(CH 3 COO) 2 4H 2 O (3.7mg, 0.015mmol) was dissolved in 2mL of methanol, placed in a 5mL small test tube, kept at 120°C for 72 hours, then cooled down to room temperature for 48 hours to obtain pink blocky crystals [Co(C 9 h 7 N 7 )(C 8 h 4 o 4 )0.75MeOH] n , yield 3.3 mg, yield 46.5% (based on L).
[0073] The compound was characterized by IR, and the results are shown in Figure 13 .
Embodiment 3
[0074] Example 3: Synthesis of Co-MOF-2
[0075] L (3.0mg, 0.014mmol), 5-methylisophthalic acid (2.7mg, 0.015mmol) Co(CH 3 COO) 2 4H 2 O (3.7mg, 0.015mmol) was dissolved in 2mL of methanol and dimethylacetamide, the volume ratio of methanol and dimethylacetamide was (1-2): (1-2), placed in a 5mL small test tube, 120 Keep the temperature at ℃ for 72 hours, then cool down to room temperature for two days after 48 hours to get pink blocky crystals [Co 2 (C 9 h 7 N 7 ) 2 (C 9 h 6 o 4 ) 2 ] n , the yield was 3.1 mg, and the yield was 41% (based on L).
[0076] The compound was characterized by IR, and the results are shown in Figure 14 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com