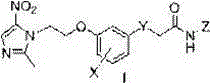
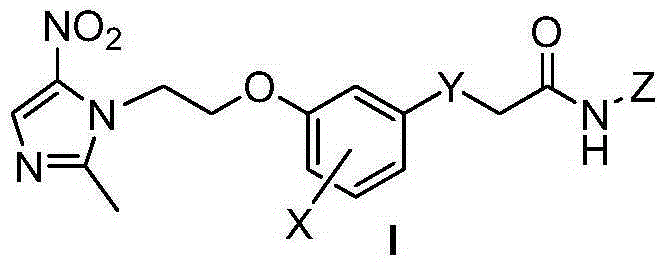
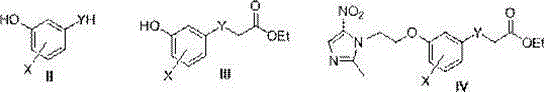Aryl connected metronidazole-amide type urease inhibitor, as well as synthesis and application thereof
A technology of metronidazole and aryl, applied in the field of preparation of anti-gastritis and gastric ulcer drugs, can solve the problems of hindering application, low activity, large dosage, etc., and achieve a good effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of 2-(5-(O-metronidazol-2'-yl)-3-hydroxyphenylthio)acetohydroxamic acid (5)
[0022] Put 28.5g of 3,5-dihydroxythiophenol, 10g of NaOH, and 15mL of ethyl bromoacetate into a 100mL round-bottomed flask, add 50mL of DMSO, stir at 60°C for 16h, pour 20mL of distilled water into it, extract it with AcOEt, and wash the organic layer with Washed 3 times with saturated brine, anhydrous MgSO 4 Dry, evaporate the solvent, purify by silica gel column chromatography, volume ratio of eluent: AcOEt:petroleum ether=1:6, and obtain 21.5 g of ethyl 2-(3,5-dihydroxyphenylthio)acetate in yellow oily liquid. Dissolve 10.5g of ethyl 2-(3,5-dihydroxyphenylthio)acetate and 10g of metronidazole chloride in 50mL of absolute ethanol, 8g of anhydrous potassium carbonate, stir at room temperature for 0.5h, and heat to 60°C for 5h , add 30mL distilled water after distilling off ethanol, extract with AcOEt, combine organic layers, anhydrous MgSO 4 Dry, evaporate the solvent...
Embodiment 2
[0024] The aryl-linked metronidazole-amide derivative series compounds 1-72 listed in Table 1 were synthesized in the same manner as in Example 1, using different substituted forms of benzaldehyde as raw materials.
[0025] Each X, Y, Z group of aryl-linked metronidazole-amide derivative series compound in the general formula I of table 1
[0026]
[0027]
[0028]
[0029]
[0030] Note: The initial raw materials were purchased from aldrich company
Embodiment 3
[0031] Embodiment 3: the inhibitory enzyme activity of compound
[0032] Add 25 μL of Jackbean urease (4U) and 25 μL (1 mM) of the test compound solution to the 96-well plate, incubate at 37 °C for 2 h, then add 55 μL of phosphate buffer containing 100 mM urea and 100 mM, and incubate at 30 °C After incubating for 15 min, add 45 μL of phenol reagent (mixed solution containing 1% phenol and 0.005% sodium nitroprusside) and 70 μL alkali reagent (mixed solution of NaOCl containing 0.5% NaOH and 0.1% active chlorine), and place at room temperature After 50 minutes, measure the OD value at 630nm with a microplate reader, and the percentage inhibition rate is calculated according to the following formula:
[0033]
[0034] All experiments were carried out in solutions at pH 7.8 (0.01M K 2 HPO 4 , 1mM EDTA, 0.01M LiCl), the level of activity is measured by the half-inhibition rate IC 50 to indicate that the IC 50 The smaller the value, the higher the activity of the compound. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com