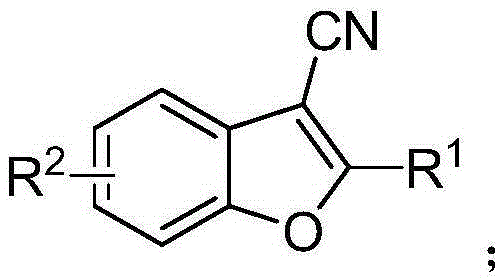
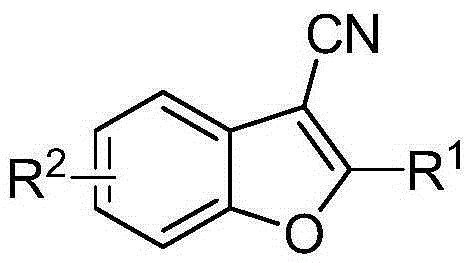
A synthetic method of 2-phenyl-3-cyano benzofuran compounds
A synthesis method and compound technology are applied in the field of cyanosynthesis of organic compounds, which can solve the problems of low selectivity and waste of metallic copper, and achieve the effects of easy availability of reaction raw materials, reduction of processes, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0021] Example R 1 R 2 1 Ph 5-Me-Ph 2 4-Me-Ph 5-Me-Ph 3 4-OMe-Ph 5-Me-Ph 4 4-Cl-Ph 5-Me-Ph 5 4-F-Ph 5-Me-Ph 6 3-Cl-Ph 5-Me-Ph 7 1-Naphthyl 5-Me-Ph 8 2-thiophene 5-Me-Ph 9 Ph Ph 10 Ph 5-Cl-Ph
Embodiment 1
[0023] Add 5-methylsalicylaldehyde (0.5mmol), Cu(OAc) to the reaction flask in turn at room temperature (20-35°C) 2 (0.1mmol), phenylacetonitrile (0.75mmol), sodium methoxide (2mmol) and DMSO (2ml), then stirred and heated to 100°C until the reaction of 5-methyl salicylaldehyde was complete. After the reaction, the reaction solution was cooled to room temperature, then added to 20ml of water, extracted three times with dichloromethane, using 10ml of dichloromethane each time, separated by silica gel chromatography column, and distilled under reduced pressure. The yield was 74%, and the identification result was: Whitesolid, mp112–113℃. 1 HNMR (400MHz, CDCl 3 ): δ8.16(d, J=7.6Hz, 2H), 7.57–7.46(m, 4H), 7.43(d, J=8.4Hz, 1H), 7.20(d, J=8.4Hz, 1H), 2.47 (s,3H). 13 CNMR (100MHz, CDCl 3 ): δ161.6, 151.7, 134.5, 131.0, 129.1, 127.9, 127.7, 127.3, 126.4, 119.62, 114.5, 111.2, 87.7, 21.32. HRMS: cacld.forC 16 h 11 NO[M + ], 233.0841; found: 233.0845.
Embodiment 2
[0025] Add 5-methylsalicylaldehyde (0.5mmol), Cu(OAc) to the reaction flask in turn at room temperature (20-35°C) 2 (0.1mmol), p-tolueneacetonitrile (0.75mmol), sodium methoxide (2mmol) and DMSO (2ml), then stirred and heated to 100°C until the reaction of 5-methyl salicylaldehyde was complete. After the reaction, the reaction solution was cooled to room temperature, then added to 20ml of water, extracted three times with dichloromethane, using 10ml of dichloromethane each time, separated by silica gel chromatography column, and distilled under reduced pressure. The yield was 56%, and the identification result was: Whitesolid, mp136–137℃. 1 HNMR (400MHz, CDCl 3 ): δ8.06(d, J=8.4Hz, 2H), 7.48–7.45(m, 1H), 7.42(d, J=8.8Hz, 1H), 7.33(d, J=8.0Hz, 2H), 7.19 (dd,J=8.4,1.2Hz,1H),2.48(s,3H),2.43(s,3H). 13 CNMR (100MHz, CDCl 3 ): δ162.0, 151.6, 141.6, 134.4, 129.8, 127.4, 126.4, 125.2, 119.5, 114.7, 111.1, 87.0, 21.6, 21.3. HRMS: cacld.forC 17 h 13 NO[M + ], 247.0997; found: 247...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com